Question: 3. A solid sample of PH3BCl3(s) is placed in a closed vessel at 80C and allow to reach equilibrium. The reaction is given below: PH3BCl3(s)PH3(g)+BCl3(g)Kp=1.57
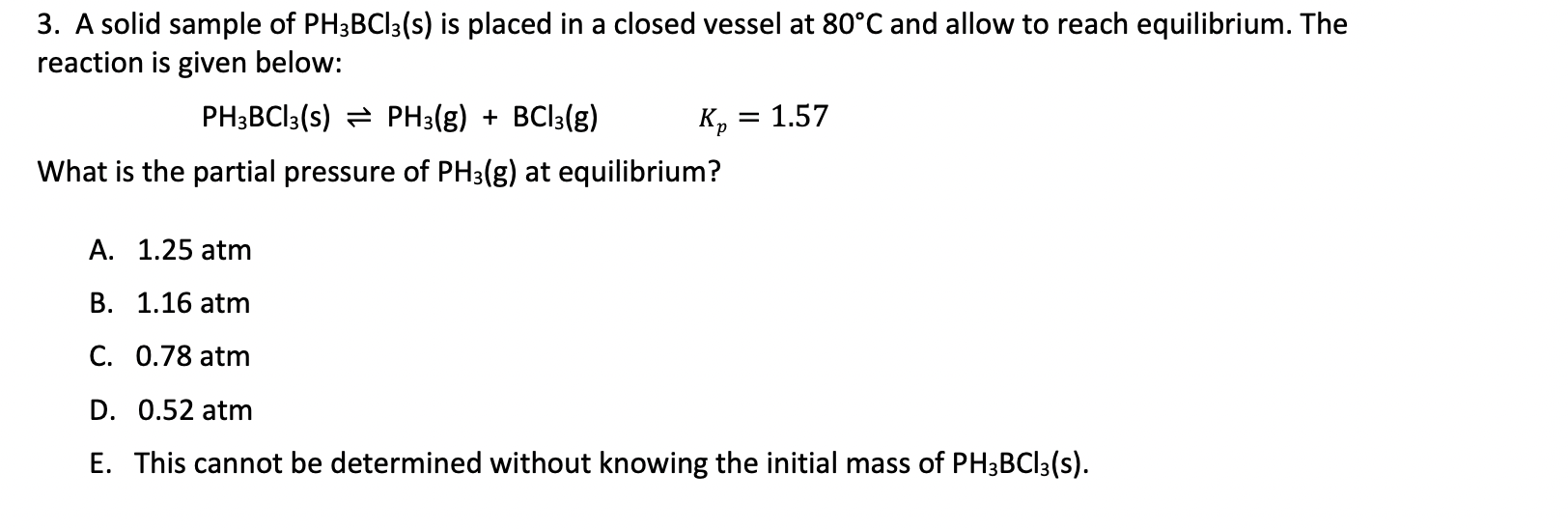
3. A solid sample of PH3BCl3(s) is placed in a closed vessel at 80C and allow to reach equilibrium. The reaction is given below: PH3BCl3(s)PH3(g)+BCl3(g)Kp=1.57 What is the partial pressure of PH3(g) at equilibrium? A. 1.25atm B. 1.16atm C. 0.78atm D. 0.52atm E. This cannot be determined without knowing the initial mass of PH3BCl3(s)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


