Question: 3. An AO process is used to remove phosphorus, nitrogen and carbon in municipal wastewater. The SRT of the process is 10 days and the
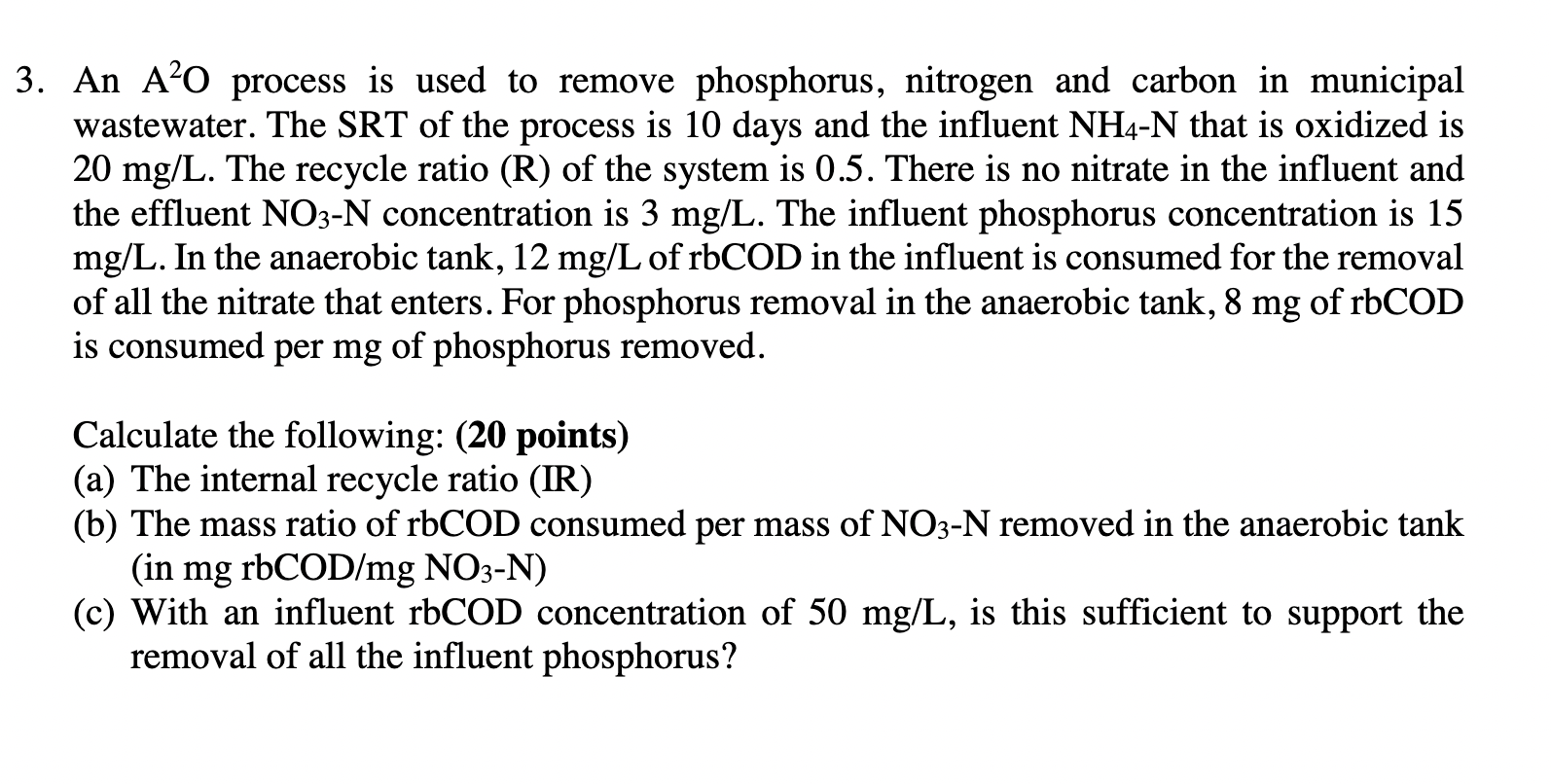
3. An AO process is used to remove phosphorus, nitrogen and carbon in municipal wastewater. The SRT of the process is 10 days and the influent NH4-N that is oxidized is 20 mg/L. The recycle ratio (R) of the system is 0.5. There is no nitrate in the influent and the effluent NO3-N concentration is 3 mg/L. The influent phosphorus concentration is 15 mg/L. In the anaerobic tank, 12 mg/L of rbCOD in the influent is consumed for the removal of all the nitrate that enters. For phosphorus removal in the anaerobic tank, 8 mg of rbCOD is consumed per mg of phosphorus removed. > Calculate the following: (20 points) (a) The internal recycle ratio (IR) (b) The mass ratio of rbCOD consumed per mass of NO3-N removed in the anaerobic tank (in mg rbCOD/mg NO3-N) (c) With an influent rbCOD concentration of 50 mg/L, is this sufficient to support the removal of all the influent phosphorus? 3. An AO process is used to remove phosphorus, nitrogen and carbon in municipal wastewater. The SRT of the process is 10 days and the influent NH4-N that is oxidized is 20 mg/L. The recycle ratio (R) of the system is 0.5. There is no nitrate in the influent and the effluent NO3-N concentration is 3 mg/L. The influent phosphorus concentration is 15 mg/L. In the anaerobic tank, 12 mg/L of rbCOD in the influent is consumed for the removal of all the nitrate that enters. For phosphorus removal in the anaerobic tank, 8 mg of rbCOD is consumed per mg of phosphorus removed. > Calculate the following: (20 points) (a) The internal recycle ratio (IR) (b) The mass ratio of rbCOD consumed per mass of NO3-N removed in the anaerobic tank (in mg rbCOD/mg NO3-N) (c) With an influent rbCOD concentration of 50 mg/L, is this sufficient to support the removal of all the influent phosphorus
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


