Question: 3) Categorize the following six as an ionic compound or a molecular (covalent) compound. Hint: what is the key distinguishing feature of an ionic compound?
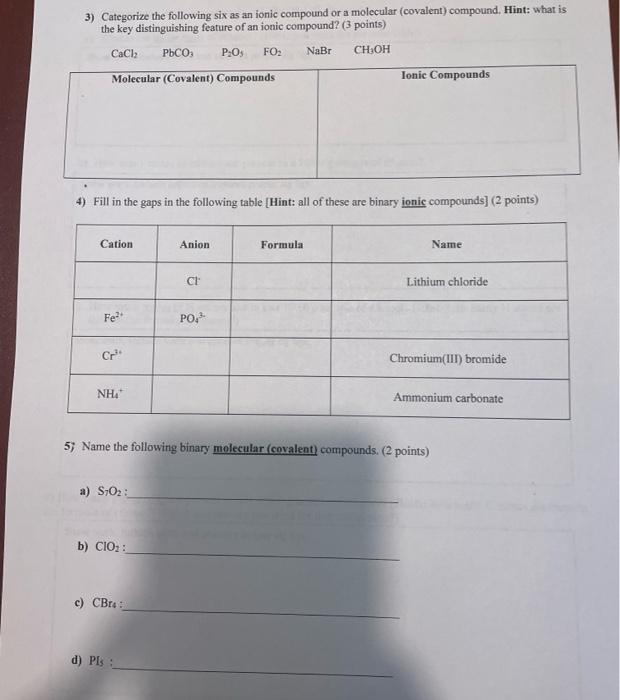
3) Categorize the following six as an ionic compound or a molecular (covalent) compound. Hint: what is the key distinguishing feature of an ionic compound? (3 points) CaCl2 P, P:03 FO: NaBr CHOH Molecular (Covalent) Compounds Ionic Compounds 4) Fill in the gaps in the following table (Hint: all of these are binary ionic compounds) (2 points) Cation Anion Formula Name C Lithium chloride Fe PO Cr? Chromium(III) bromide NHA Ammonium carbonate 5) Name the following binary molecular (covalent) compounds. (2 points) a) S:02: b) CIO: c) CBr d) PIS
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


