Question: 3. Complete the table below. It contains information that you will use in Part 1 of the experi- ment. Since [Fe+] >> [SCN-], you
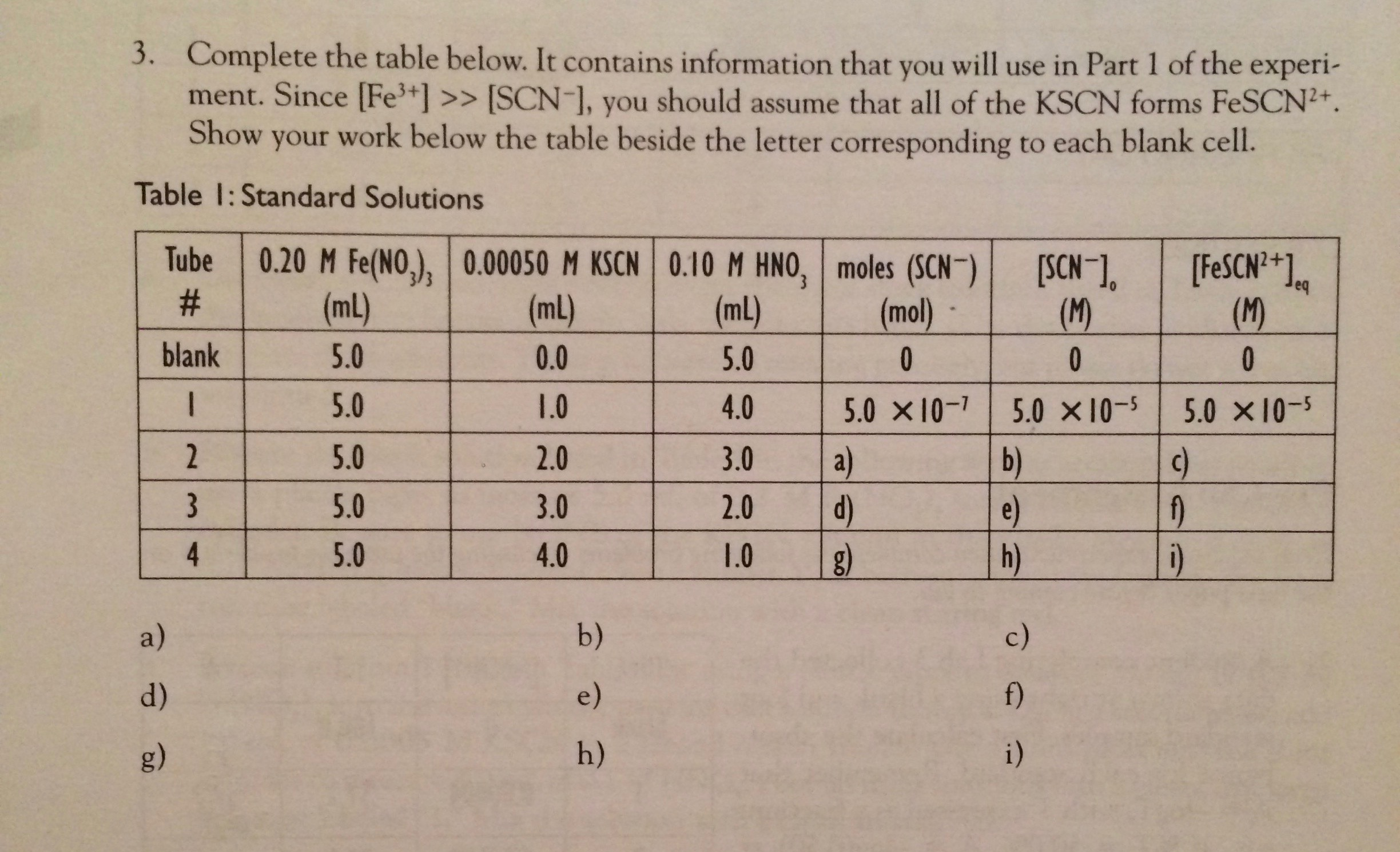
3. Complete the table below. It contains information that you will use in Part 1 of the experi- ment. Since [Fe+] >> [SCN-], you should assume that all of the KSCN forms FeSCN+. Show your work below the table beside the letter corresponding to each blank cell. Table 1: Standard Solutions Tube 0.20 M Fe(NO), 0.00050 M KSCN 0.10 M HNO, moles (SCN) # (mL) (mL) (mL) 5.0 0.0 5.0 5.0 1.0 4.0 5.0 5.0 5.0 blank 1 2 a) d) g) 3 4 2.0 3.0 4.0 b) e) h) 3.0 2.0 1.0 (mol) 0 5.0 10-7 a) d) g) [SCN-]. (M) 0 5.0 10-5 b) e) h) c) f) i) [FeSCN+1 (M) 0 5.0 10-5 c) f) i)
Step by Step Solution
3.45 Rating (165 Votes )
There are 3 Steps involved in it
a it must be twice as SCNin tube1 since it has double volume of ... View full answer

Get step-by-step solutions from verified subject matter experts


