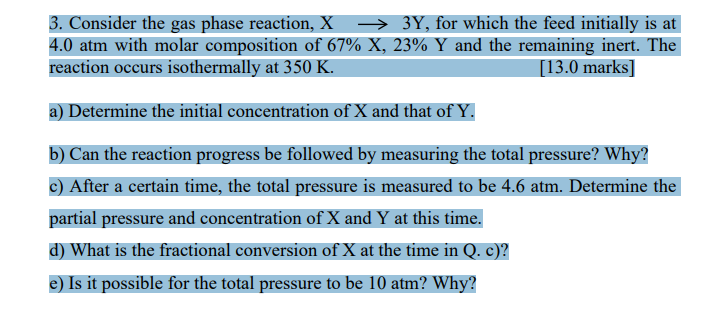
Question: 3 . Consider the gas phase reaction, X 3 Y , for which the feed initially is at 4 . 0 atm with molar composition
Consider the gas phase reaction, X Y for which the feed initially is at
atm with molar composition of X Y and the remaining inert. The
reaction occurs isothermally at K marks
a Determine the initial concentration of X and that of Y
b Can the reaction progress be followed by measuring the total pressure? Why?
c After a certain time, the total pressure is measured to be atm. Determine the
partial pressure and concentration of X and Y at this time.
d What is the fractional conversion of X at the time in Q c
e Is it possible for the total pressure to be atm? Why?
Consider the gas phase reaction, for which the feed initially is at
atm with molar composition of and the remaining inert. The
reaction occurs isothermally at
marks
a Determine the initial concentration of and that of
b Can the reaction progress be followed by measuring the total pressure? Why?
c After a certain time, the total pressure is measured to be atm. Determine the
partial pressure and concentration of and at this time.
d What is the fractional conversion of at the time in
e Is it possible for the total pressure to be atm Why?
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


