Question: Problem 1 Calculate the overall conversion (X = FAO-FA/FAO) in the following recycle reactor when the volume of reactor equals two m for the gas
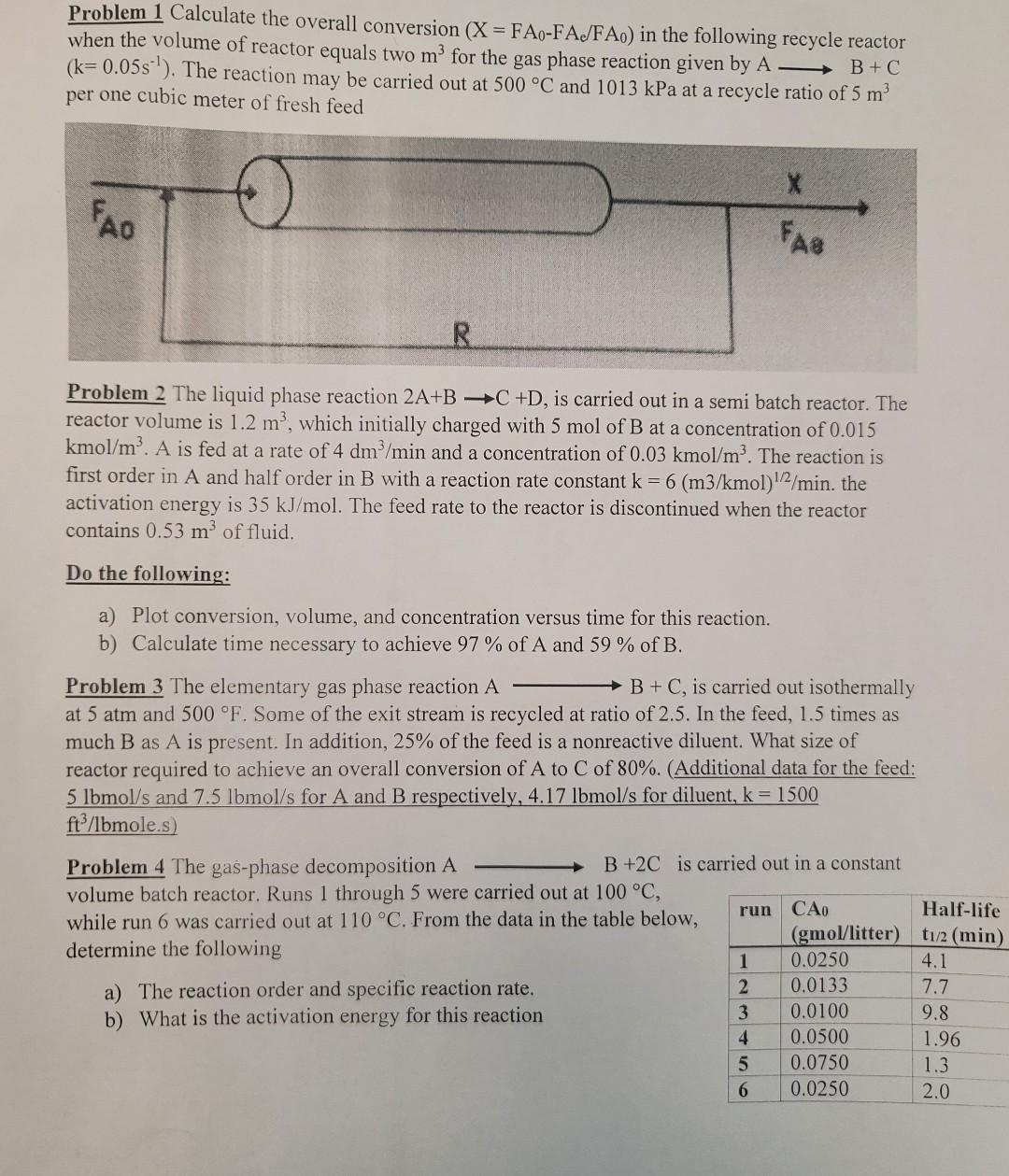
Problem 1 Calculate the overall conversion (X = FAO-FA/FAO) in the following recycle reactor when the volume of reactor equals two m for the gas phase reaction given by A - B+C (k=0.05s'). The reaction may be carried out at 500 C and 1013 kPa at a recycle ratio of 5 m? per one cubic meter of fresh feed o X FAO FAQ R Problem 2 The liquid phase reaction 2A+B C +D, is carried out in a semi batch reactor. The reactor volume is 1.2 m', which initially charged with 5 mol of B at a concentration of 0.015 kmol/m. A is fed at a rate of 4 dm /min and a concentration of 0.03 kmol/m. The reaction is first order in A and half order in B with a reaction rate constant k = 6 (m3/kmol) 1/2/min. the activation energy is 35 kJ/mol. The feed rate to the reactor is discontinued when the reactor contains 0.53 m of fluid. Do the following: a) Plot conversion, volume, and concentration versus time for this reaction. b) Calculate time necessary to achieve 97 % of A and 59 % of B. Problem 3 The elementary gas phase reaction A B+C, is carried out isothermally at 5 atm and 500 F. Some of the exit stream is recycled at ratio of 2.5. In the feed, 1.5 times as much B as A is present. In addition, 25% of the feed is a nonreactive diluent. What size of reactor required to achieve an overall conversion of A to C of 80%. (Additional data for the feed: 5 lbmol/s and 7.5 lbmol/s for A and B respectively, 4.17 lbmol/s for diluent, k = 1500 ftp/lbmole.s) Problem 4 The gas-phase decomposition A B +2C is carried out in a constant volume batch reactor. Runs 1 through 5 were carried out at 100 C, run CAO Half-life while run 6 was carried out at 110 C. From the data in the table below, (gmol/litter) t1/2 (min) determine the following 1 0.0250 4.1 a) The reaction order and specific reaction rate. 0.0133 7.7 b) What is the activation energy for this reaction 3 0.0100 9.8 4 0.0500 1.96 5 0.0750 1.3 6 0.0250 2.0 N
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


