Question: 3. Explain: Based on your knowledge of table salt and of Gibb's Free Energy which of hese two reactions would you expect to have a
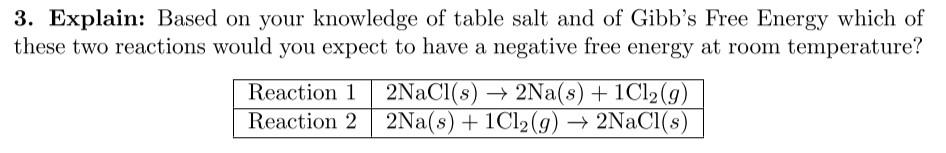
3. Explain: Based on your knowledge of table salt and of Gibb's Free Energy which of hese two reactions would you expect to have a negative free energy at room temperature
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


