Question: 3 FeO + 2 A l = A l 2 O 3 + 3 F e Bir pota firininda % 2 2 . 4 C
FeO
Bir pota firininda ve den ibaret gaz yakit de haver le yah
a gazn kalorifik gucanu
o oluan alevin scaklgn bulunuz P
Fanma reaksiyonu:
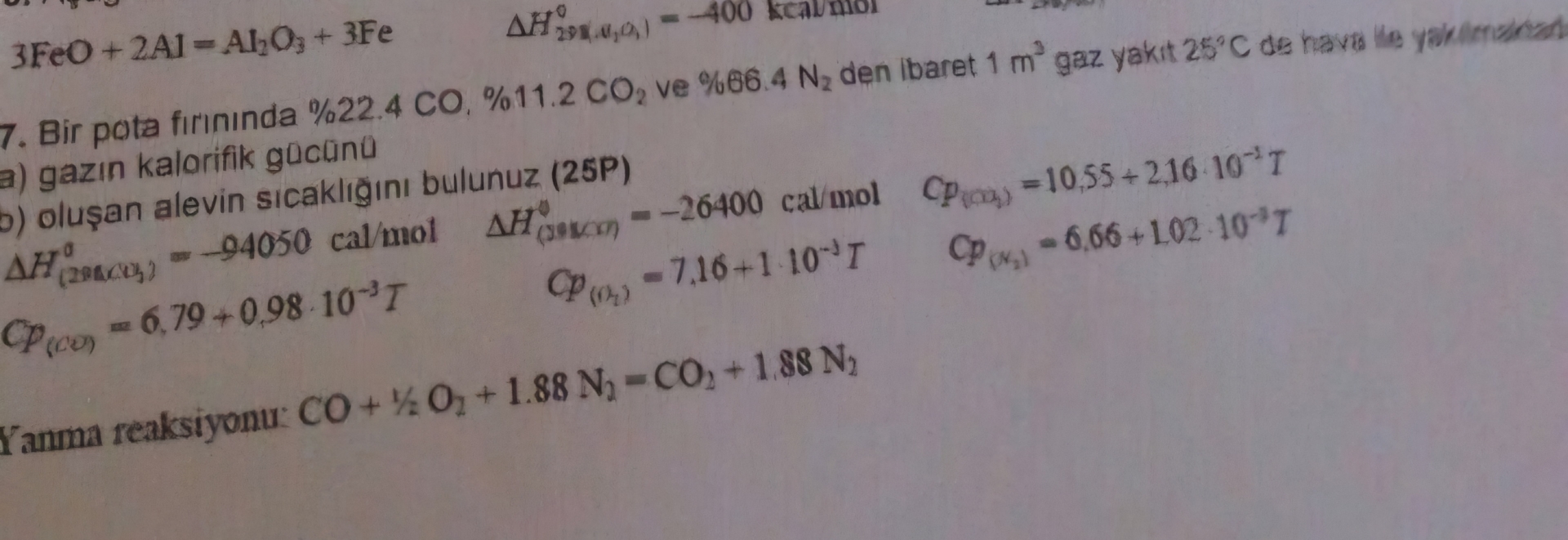
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


