Question: 3. For the following compound, how many aromatic rings does it contain, how many pi electron pairs are part of its conjugated system, and how
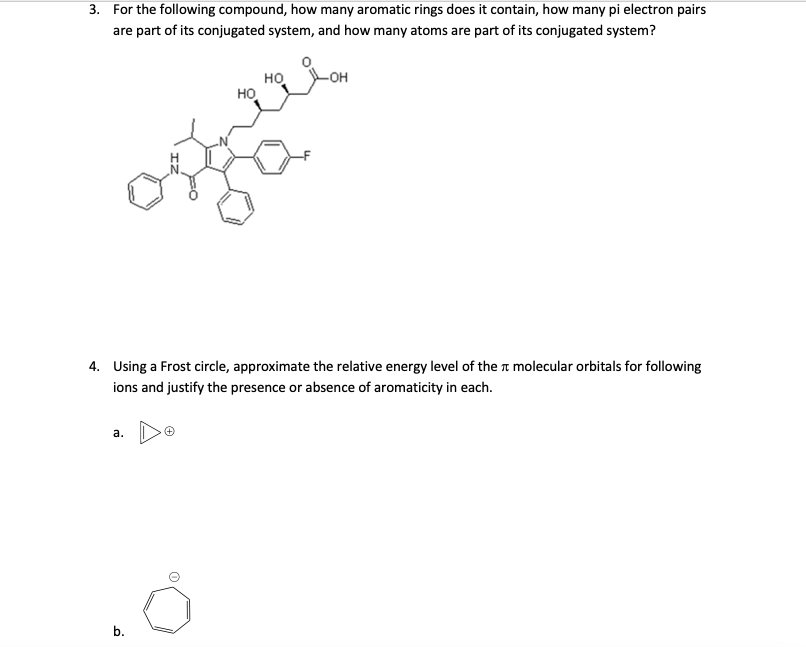
3. For the following compound, how many aromatic rings does it contain, how many pi electron pairs are part of its conjugated system, and how many atoms are part of its conjugated system? -OH HO HO mie 4. Using a Frost circle, approximate the relative energy level of the a molecular orbitals for following ions and justify the presence or absence of aromaticity in each. a. 0 b
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


