Question: 3. Hexane, shown below, has very poor solubility in water (9mg/L). hexane 2-hexanol 1,6-hexanediol a. Based on what you know about the hydrophobic effect, explain,
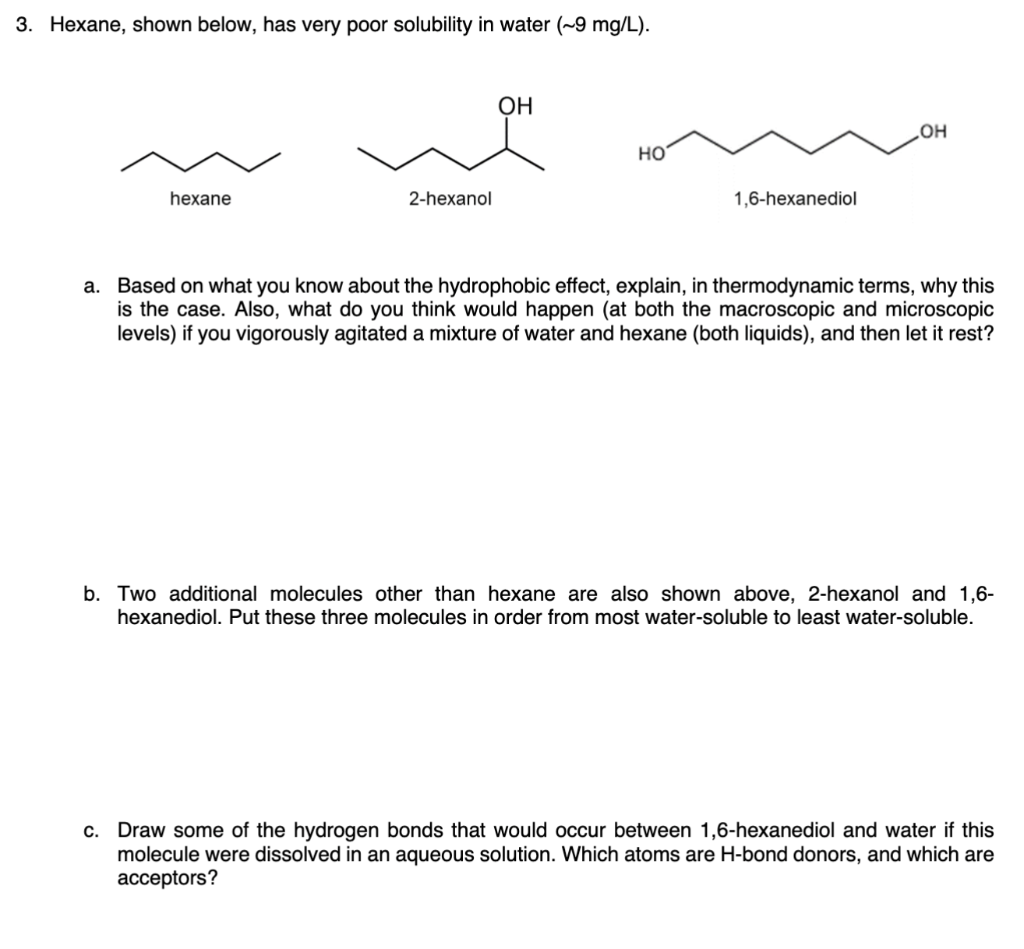
3. Hexane, shown below, has very poor solubility in water (9mg/L). hexane 2-hexanol 1,6-hexanediol a. Based on what you know about the hydrophobic effect, explain, in thermodynamic terms, why this is the case. Also, what do you think would happen (at both the macroscopic and microscopic levels) if you vigorously agitated a mixture of water and hexane (both liquids), and then let it rest? b. Two additional molecules other than hexane are also shown above, 2-hexanol and 1,6hexanediol. Put these three molecules in order from most water-soluble to least water-soluble. c. Draw some of the hydrogen bonds that would occur between 1,6-hexanediol and water if this molecule were dissolved in an aqueous solution. Which atoms are H-bond donors, and which are acceptors
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


