Question: 3. If 25.0 mL 0.020 M Ca(NO3)2 solution was mixed with 25.0 mL 0.010 M Na3PO4, determine whether a precipitate would spontaneously form at 5C,
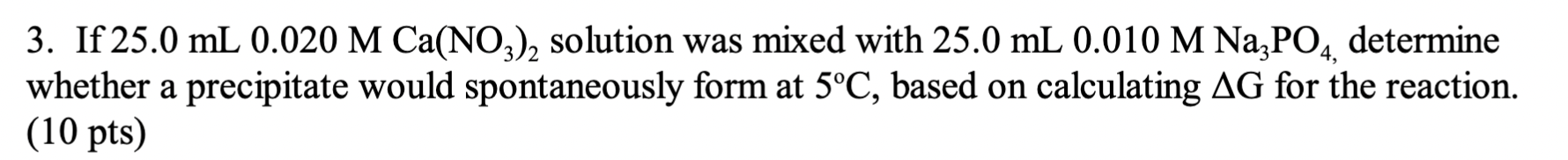
3. If 25.0 mL 0.020 M Ca(NO3)2 solution was mixed with 25.0 mL 0.010 M Na3PO4, determine whether a precipitate would spontaneously form at 5C, based on calculating AG for the reaction. (10 pts)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


