Question: Complete the following questions based on the remote laboratory procedures: 1. What are some similarities in the two reaction procedures (microscale vs miniscale)? What
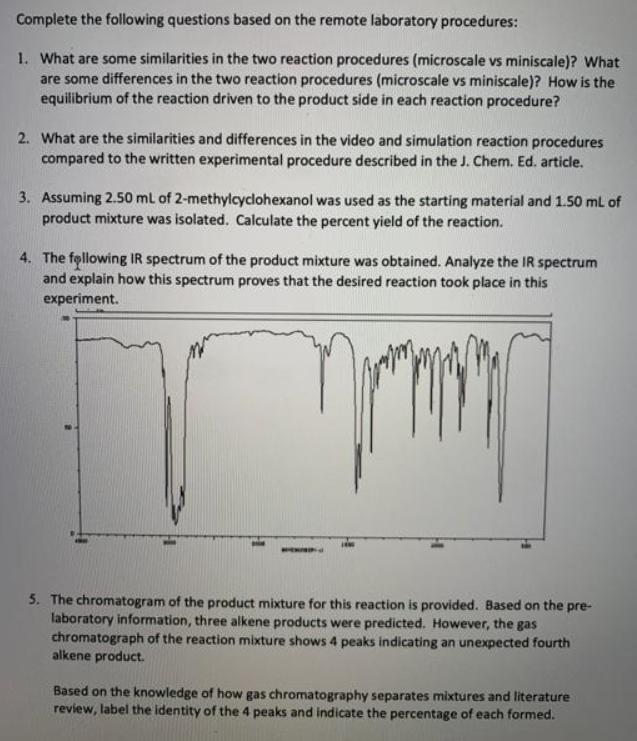
Complete the following questions based on the remote laboratory procedures: 1. What are some similarities in the two reaction procedures (microscale vs miniscale)? What are some differences in the two reaction procedures (microscale vs miniscale)? How is the equilibrium of the reaction driven to the product side in each reaction procedure? 2. What are the similarities and differences in the video and simulation reaction procedures compared to the written experimental procedure described in the J. Chem. Ed. article. 3. Assuming 2.50 ml of 2-methylcyclohexanol was used as the starting material and 1.50 mL of product mixture was isolated. Calculate the percent yield of the reaction. 4. The following IR spectrum of the product mixture was obtained. Analyze the IR spectrum and explain how this spectrum proves that the desired reaction took place in this experiment. 5. The chromatogram of the product mixture for this reaction is provided. Based on the pre- laboratory information, three alkene products were predicted. However, the gas chromatograph of the reaction mixture shows 4 peaks indicating an unexpected fourth alkene product. Based on the knowledge of how gas chromatography separates mixtures and literature review, label the identity of the 4 peaks and indicate the percentage of each formed.
Step by Step Solution
3.51 Rating (168 Votes )
There are 3 Steps involved in it
O Simitarities are iven below Borh reactios will do in laboratoy scale BoM reachion oeme in ytass eq... View full answer

Get step-by-step solutions from verified subject matter experts


