Question: 3 n 1+ 4t 6t + ... 2 03 - Show how Vg Vi behaves close to the critical temperature. it is the reduced pressure
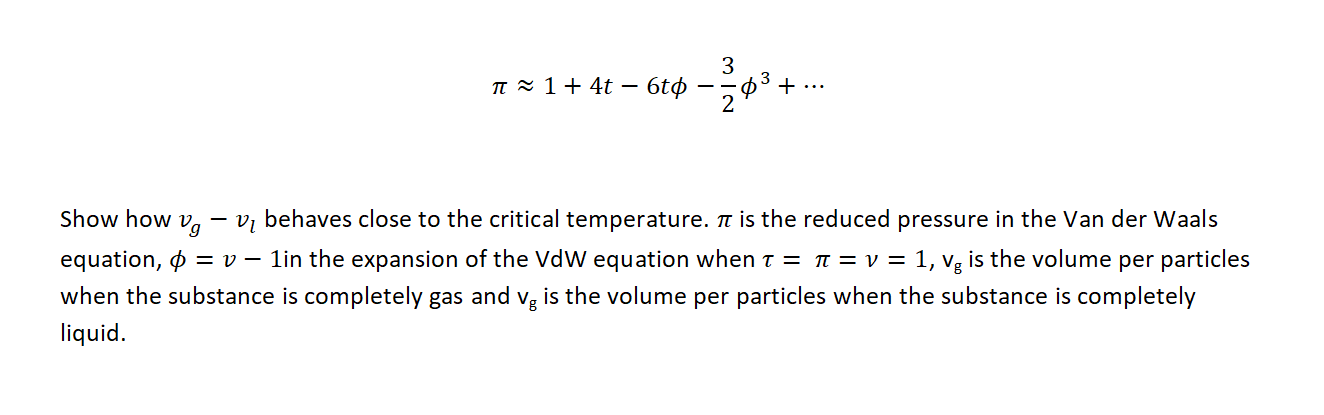
3 n 1+ 4t 6t + ... 2 03 - Show how Vg Vi behaves close to the critical temperature. it is the reduced pressure in the Van der Waals equation, 0 = 0 1in the expansion of the VdW equation when t = t = v= = 1, vg is the volume per particles when the substance is completely gas and vg is the volume per particles when the substance is completely liquid
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


