Question: 3 II 1 + 4t 6t 30 + .-* Show how Ug Vi behaves close to the critical temperature. It is the reduced pressure in
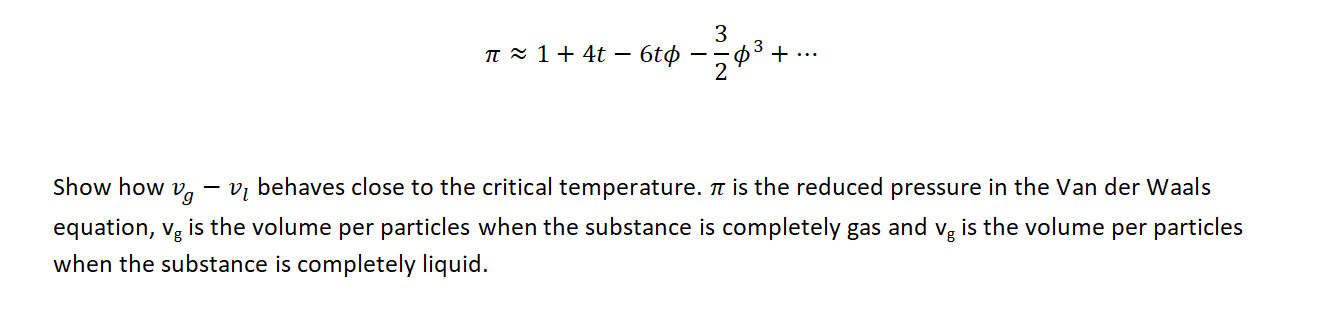
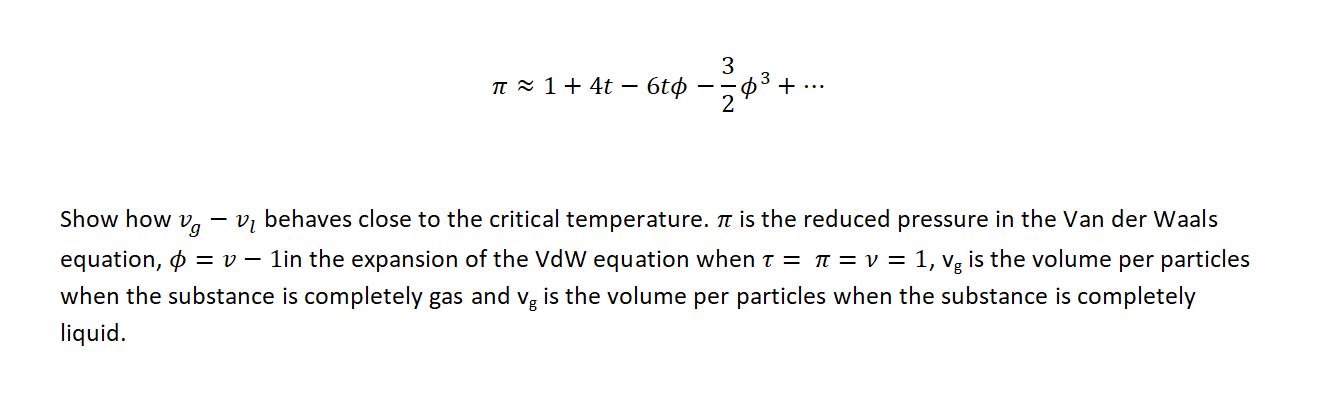
3 II 1 + 4t 6t 30 + .-* Show how Ug Vi behaves close to the critical temperature. It is the reduced pressure in the Van der Waals equation, vg is the volume per particles when the substance is completely gas and vg is the volume per particles when the substance is completely liquid. 3 n 1+ 4t 6t + ... 2 03 - Show how Vg Vi behaves close to the critical temperature. it is the reduced pressure in the Van der Waals equation, 0 = 0 1in the expansion of the VdW equation when t = t = v= = 1, vg is the volume per particles when the substance is completely gas and vg is the volume per particles when the substance is completely liquid. 3 II 1 + 4t 6t 30 + .-* Show how Ug Vi behaves close to the critical temperature. It is the reduced pressure in the Van der Waals equation, vg is the volume per particles when the substance is completely gas and vg is the volume per particles when the substance is completely liquid. 3 n 1+ 4t 6t + ... 2 03 - Show how Vg Vi behaves close to the critical temperature. it is the reduced pressure in the Van der Waals equation, 0 = 0 1in the expansion of the VdW equation when t = t = v= = 1, vg is the volume per particles when the substance is completely gas and vg is the volume per particles when the substance is completely liquid
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


