Question: 3 part Enter your answer in the provided box. How many liters of gaseous hydrogen bromide at 65C and 0.663atm will a chemist need if
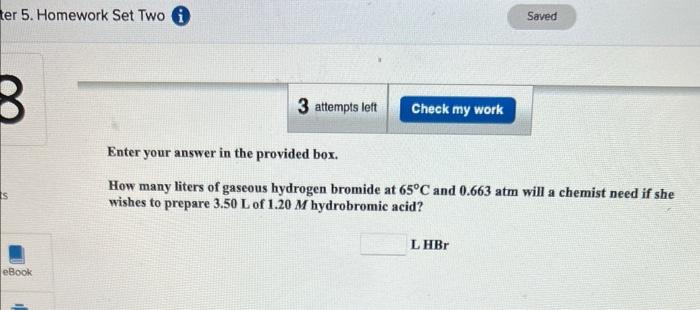
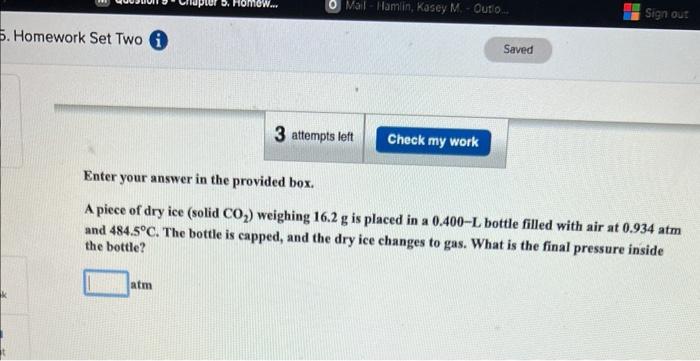
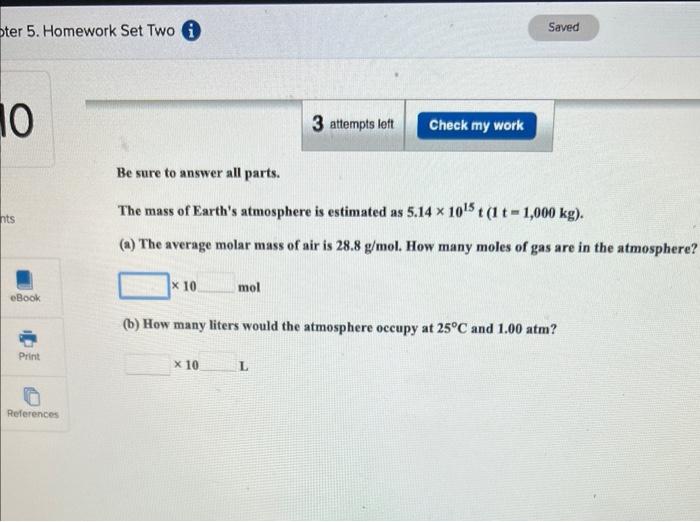
Enter your answer in the provided box. How many liters of gaseous hydrogen bromide at 65C and 0.663atm will a chemist need if she wishes to prepare 3.50L of 1.20M hydrobromic acid? Enter your answer in the provided box. A piece of dry ice (solid CO2 ) weighing 16.2g is placed in a 0.400L bottle filled with air at 0.934atm and 484.5C. The bottle is capped, and the dry ice changes to gas. What is the final pressure inside the bottle? atm Be sure to answer all parts. The mass of Earth's atmosphere is estimated as 5.141015t(1t=1,000kg). (a) The average molar mass of air is 28.8g/mol. How many moles of gas are in the atmosphere? 10mol (b) How many liters would the atmosphere occupy at 25C and 1.00atm ? 10L
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


