Question: 3 Please try the problem again, your previous tries are listed below. An aquecus salt solution is formed by adding 0.897mol Iron (III) nitrate to
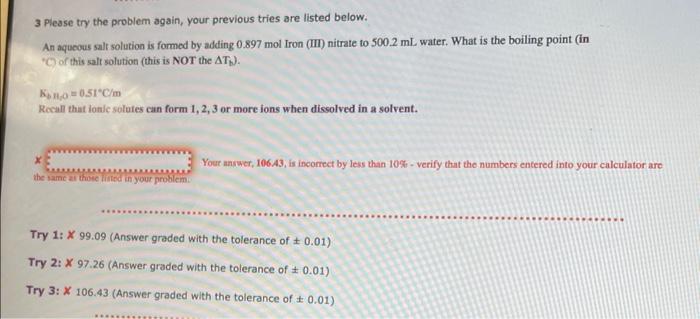
3 Please try the problem again, your previous tries are listed below. An aquecus salt solution is formed by adding 0.897mol Iron (III) nitrate to 500.2mL water. What is the boiling point (in 'C) of this salt solution (this is NOT the Tb ). KbH,O=0.5tC/m Rocall that ionle solutes can form 1,2,3 or more ions when dissolved in a solvent. x Your answer, 106. A3, is incorrect by less than 10% - verify that the numbers entered into your calculator are the same ar those nirted in your problem. Try 1: X99.09 (Answer graded with the tolerance of 0.01 ) Try 2: 97.26 (Answer graded with the tolerance of 0.01 ) Try 3: X106.43 (Answer graded with the tolerance of 0.01 )
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


