Question: 4 Please try the problem again, your previous tries are listed below. An aqueous salt solution is formed by adding 11.67g sodium sulfate (solute) to
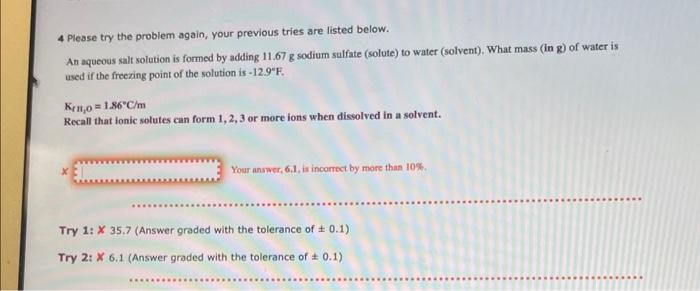
4 Please try the problem again, your previous tries are listed below. An aqueous salt solution is formed by adding 11.67g sodium sulfate (solute) to water (solvent). What mass (in g) of water is used if the freezing point of the solution is 12.9F. KtHo=1.86C/m Recall that ionic solutes can form 1,2,3 or more ions when dissolved in a solvent. Your answer, 6.1, is incorrect by more than 10%. Try 1: 35.7 (Answer graded with the tolerance of 0.1) Try 2: 6.1 (Answer graded with the tolerance of 0.1 )
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


