Question: 3. State the substances undergoing oxidation and reduction in the following ionic redox reactions. (a) Al(s)+Cr3+(aq)Al3+(aq)+Cr(s) (b) F2(g)+2Cl(aq)2F(aq)+Cl2(g) 23. Write a balanced half-reaction for each

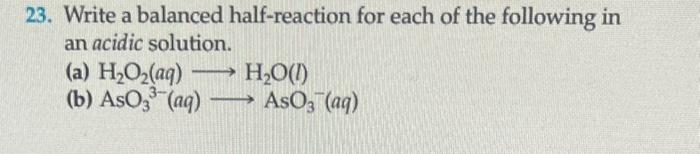
3. State the substances undergoing oxidation and reduction in the following ionic redox reactions. (a) Al(s)+Cr3+(aq)Al3+(aq)+Cr(s) (b) F2(g)+2Cl(aq)2F(aq)+Cl2(g) 23. Write a balanced half-reaction for each of the following in an acidic solution. (a) H2O2(aq)H2O(l) (b) AsO33(aq)AsO3(aq)
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


