Question: 3. The dimer, M2, diffuses at steady state from bulk solution to a catalytic surface, where it dissociates instantaneously to form 2M. Species A then
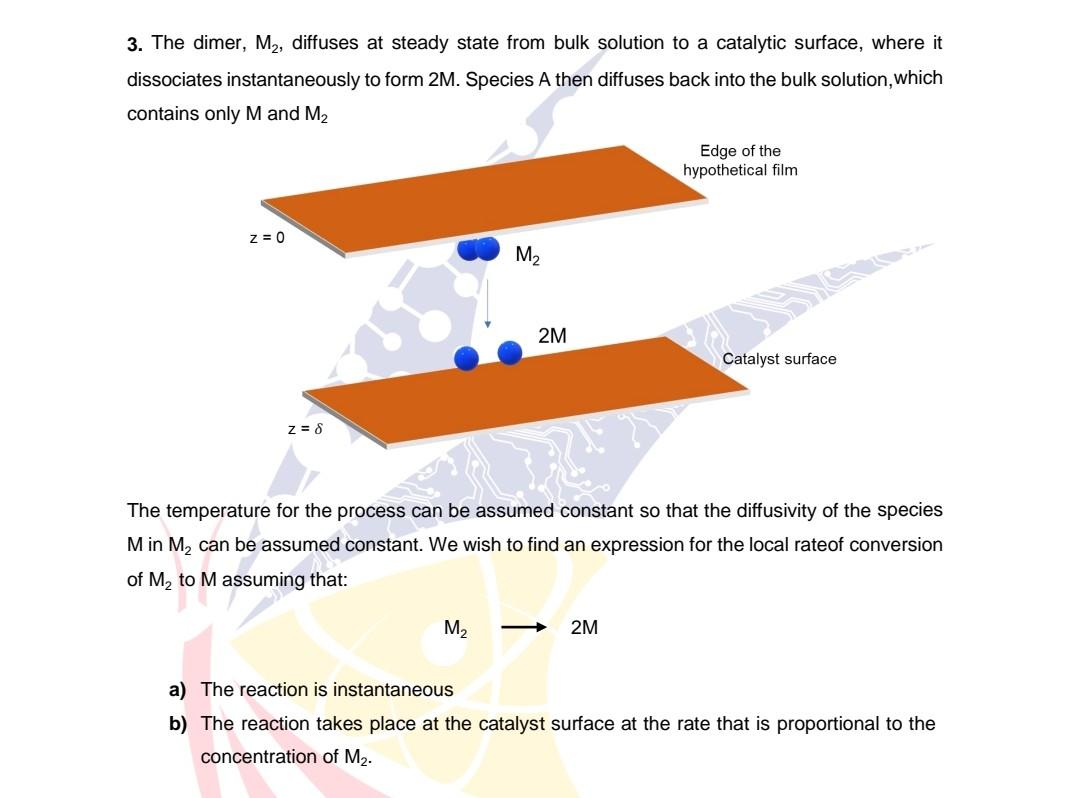
3. The dimer, M2, diffuses at steady state from bulk solution to a catalytic surface, where it dissociates instantaneously to form 2M. Species A then diffuses back into the bulk solution, which contains only M and M2 The temperature for the process can be assumed constant so that the diffusivity of the species M in M2 can be assumed constant. We wish to find an expression for the local rateof conversion of M2 to M assuming that: a) The reaction is instantar b) The reaction takes place at the catalyst surface at the rate that is proportional to the concentration of M2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


