Question: (3) Uranium Energy Content Problem: For this possible nuclear decay outcome during the fission process, a) Compute the reaction energy in MeV per reaction given
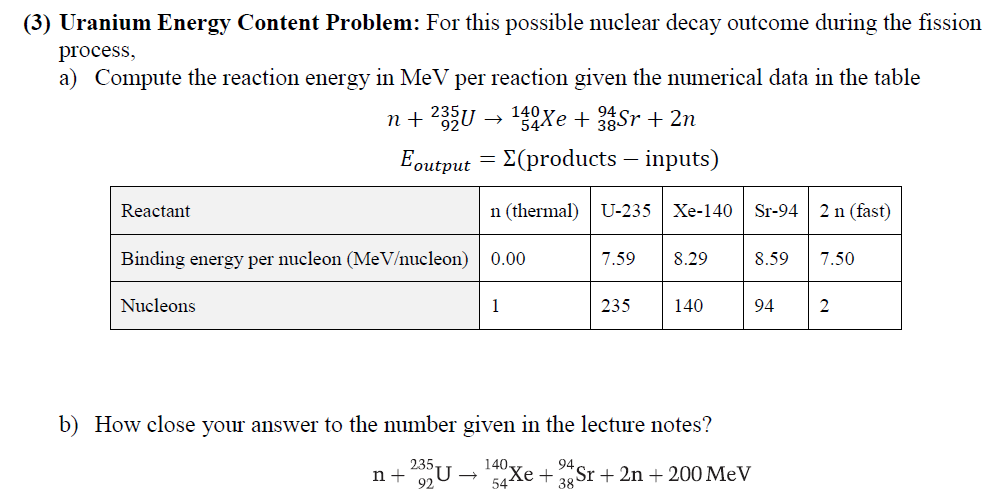
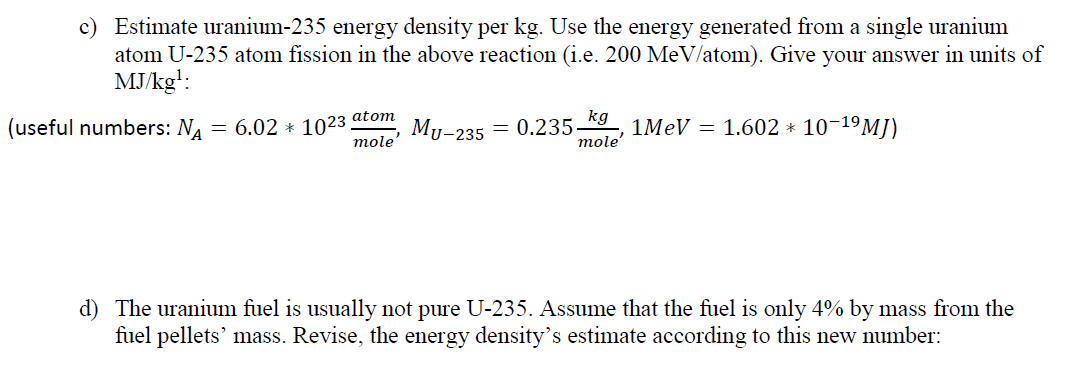
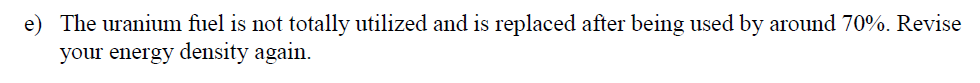
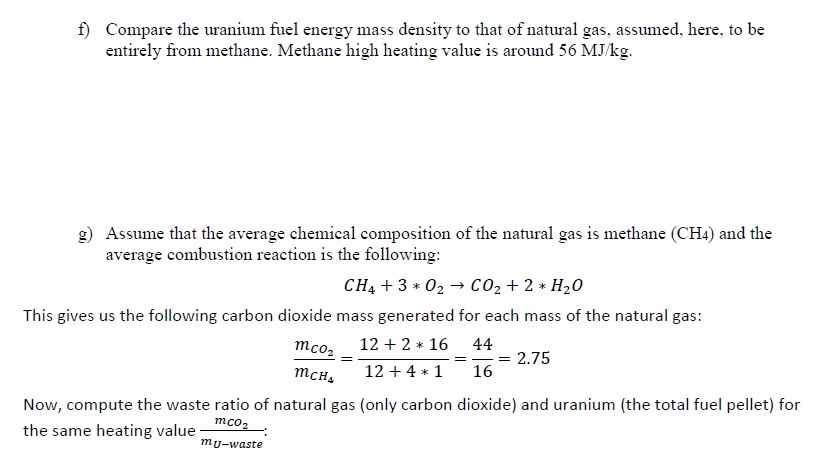

(3) Uranium Energy Content Problem: For this possible nuclear decay outcome during the fission process, a) Compute the reaction energy in MeV per reaction given the numerical data in the table n+235U 142xe + 33Sr + 2n Eoutput E(products inputs) Reactant n (thermal) U-2351 Xe-140 | Sr-94 2 n (fast) Binding energy per nucleon (MeVucleon) 0.00 7.59 8.29 8.59 7.50 Nucleons 1 235 140 94 2 b) How close your answer to the number given in the lecture notes? 2930 150 Xe + 34Sr + 2n + 200 MeV 94 n+ 544 38 c) Estimate uranium-235 energy density per kg. Use the energy generated from a single uranium atom U-235 atom fission in the above reaction (i.e. 200 MeV/atom). Give your answer in units of MJ/kg?: (useful numbers: NA = 6.02 * 1023 atom kg = 0.235 1MeV = 1.602 * 10-19M]) mole' mole' Mu-235 d) The uranium fuel is usually not pure U-235. Assume that the fuel is only 4% by mass from the fuel pellets' mass. Revise, the energy density's estimate according to this new number: e) The uranium fuel is not totally utilized and is replaced after being used by around 70%. Revise your energy density again. f) Compare the uranium fuel energy mass density to that of natural gas, assumed, here, to be entirely from methane. Methane high heating value is around 56 MJ/kg. g) Assume that the average chemical composition of the natural gas is methane (CH4) and the average combustion reaction is the following: CH4 + 3 + 02 CO2 + 2 * H20 This gives us the following carbon dioxide mass generated for each mass of the natural gas: 12 + 2 * 16 44 = 2.75 12 + 4 * 1 16 Now, compute the waste ratio of natural gas (only carbon dioxide) and uranium (the total fuel pellet) for the same heating value mcoz mu-waste mcoz , h) Next, let us compare the volumetric density of storing carbon dioxide as dense dry ice of 1.6 kg/liter to uranium waste of 19 kg/liter, (we are ignoring containers and shielding in this simple estimate)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


