Question: 3. Various attractive interactions can be present between molecular compounds in their liquid state: Dipole-dipole interactions, London (dispersion) forces and hydrogen bonds forces. A (5
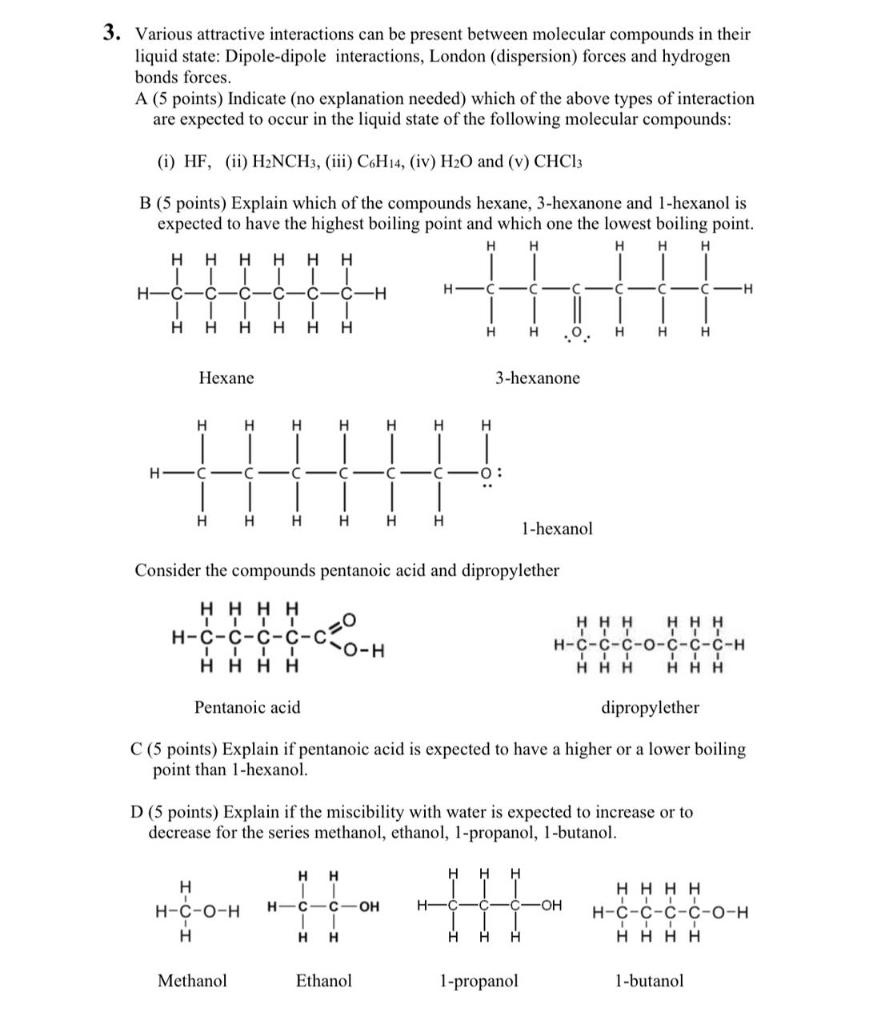
3. Various attractive interactions can be present between molecular compounds in their liquid state: Dipole-dipole interactions, London (dispersion) forces and hydrogen bonds forces. A (5 points) Indicate (no explanation needed) which of the above types of interaction are expected to occur in the liquid state of the following molecular compounds: (i) HF, (ii) H2NCH3, (iii) C6H14, (iv) H2O and (v) CHCl3 B (5 points) Explain which of the compounds hexane, 3-hexanone and 1-hexanol is expected to have the highest boiling point and which one the lowest boiling point. Hexane 3-hexanone 1-hexanol Consider the compounds pentanoic acid and dipropylether Pentanoic acid dipropylether C (5 points) Explain if pentanoic acid is expected to have a higher or a lower boiling point than 1-hexanol. D (5 points) Explain if the miscibility with water is expected to increase or to decrease for the series methanol, ethanol, 1-propanol, 1-butanol
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


