Question: 3) Write balanced molecular and net ionic equations for the following acid-base reactions HC2H3O2(aq)+KOH(aq)H2SO4(aq)+NaOH(aq)Ba(OH)2(aq)+H3PO4(aq)HClO4(aq)+Mg(OH)2(s)(allcommonperchloratesaresoluble)
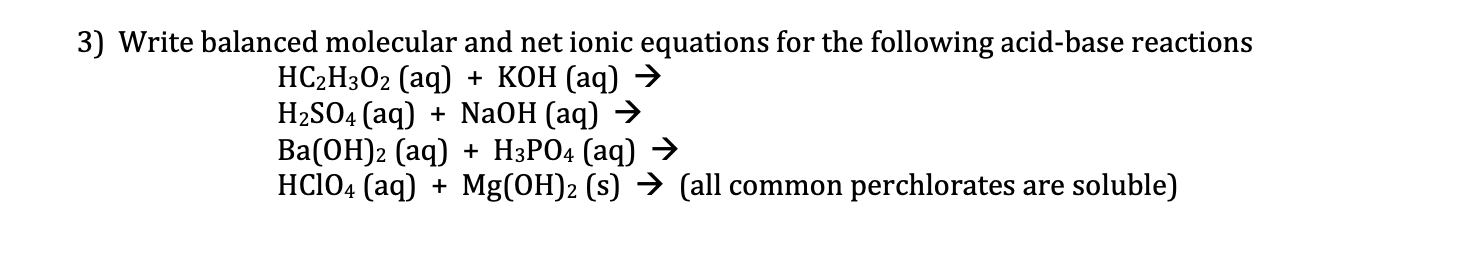
3) Write balanced molecular and net ionic equations for the following acid-base reactions HC2H3O2(aq)+KOH(aq)H2SO4(aq)+NaOH(aq)Ba(OH)2(aq)+H3PO4(aq)HClO4(aq)+Mg(OH)2(s)(allcommonperchloratesaresoluble)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


