Question: (30 points) CO2 is diffusing through N2 at atmospheric pressure (101.32kPa) and 25C in a cylindrical tube. The partial pressure of CO2 at point A
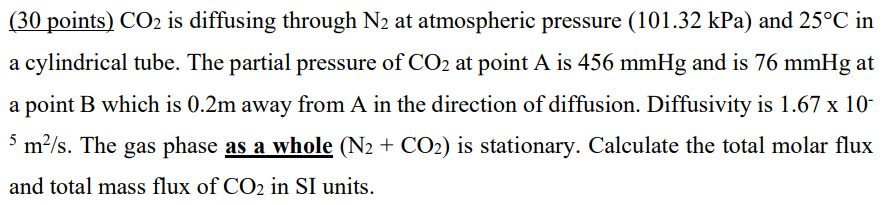
(30 points) CO2 is diffusing through N2 at atmospheric pressure (101.32kPa) and 25C in a cylindrical tube. The partial pressure of CO2 at point A is 456mmHg and is 76mmHg at a point B which is 0.2m away from A in the direction of diffusion. Diffusivity is 1.6710 5m2/s. The gas phase as a whole (N2+CO2) is stationary. Calculate the total molar flux and total mass flux of CO2 in SI units
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


