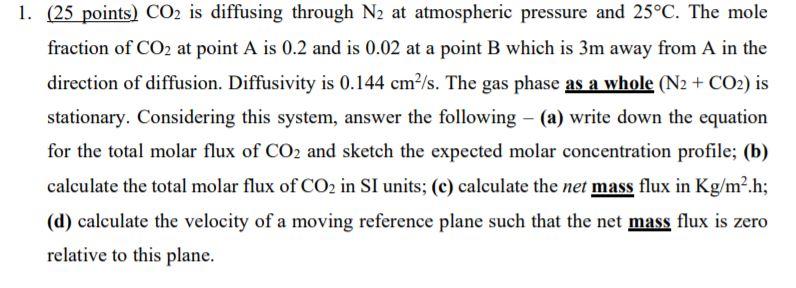
Question: I need help with B! please help 1. (25 points) CO2 is diffusing through N2 at atmospheric pressure and 25C. The mole fraction of CO2
I need help with B! please help
1. (25 points) CO2 is diffusing through N2 at atmospheric pressure and 25C. The mole fraction of CO2 at point A is 0.2 and is 0.02 at a point B which is 3m away from A in the direction of diffusion. Diffusivity is 0.144 cm-/s. The gas phase as a whole (N2 + CO2) is stationary. Considering this system, answer the following - (a) write down the equation for the total molar flux of CO2 and sketch the expected molar concentration profile; (b) calculate the total molar flux of CO2 in SI units; (e) calculate the net mass flux in Kg/m.h; (d) calculate the velocity of a moving reference plane such that the net mass flux is zero relative to this plane
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


