Question: 33 Question 33 (40 points) o El gas Temp (deg C) liquid B solid Energy Image: Graph of temp versus energy. A line slopes up
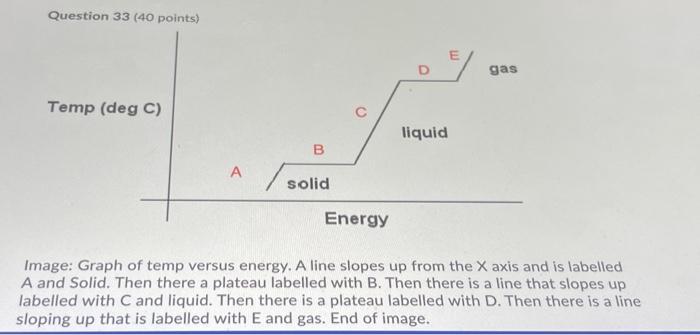

Question 33 (40 points) o El gas Temp (deg C) liquid B solid Energy Image: Graph of temp versus energy. A line slopes up from the X axis and is labelled A and Solid. Then there a plateau labelled with B. Then there is a line that slopes up labelled with C and liquid. Then there is a plateau labelled with D. Then there is a line sloping up that is labelled with E and gas. End of image. Image: Graph of temp versus energy. A line slopes up from the X axis and is labelled A and Solid. Then there a plateau labelled with B. Then there is a line that slopes up labelled with C and liquid. Then there is a plateau labelled with D. Then there is a line sloping up that is labelled with E and gas. End of image. Calculate the total energy required (In Joules) to bring 240.00 g of compound "X" (MW = 150.00 g/mol) from -60.00 degrees C to 215.00 degrees C. Data: melting point of X = 85.00 degrees C boiling point of X = 155.00 degrees C C(s) (heat capacity solid X) = 4.165 J/g/ degrees C: C) (heat capacity liquid X) = 2.703 J/g/ degrees C; (g) (heat capacity gas X) = 3.094 J/g/ degrees C; AHius (molar heat of fusion for X) = 3.706 kJ/mol AHvap (molar heat of vaporization for X) = 3.031 kJ/mol Your Answer: Answer units
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


