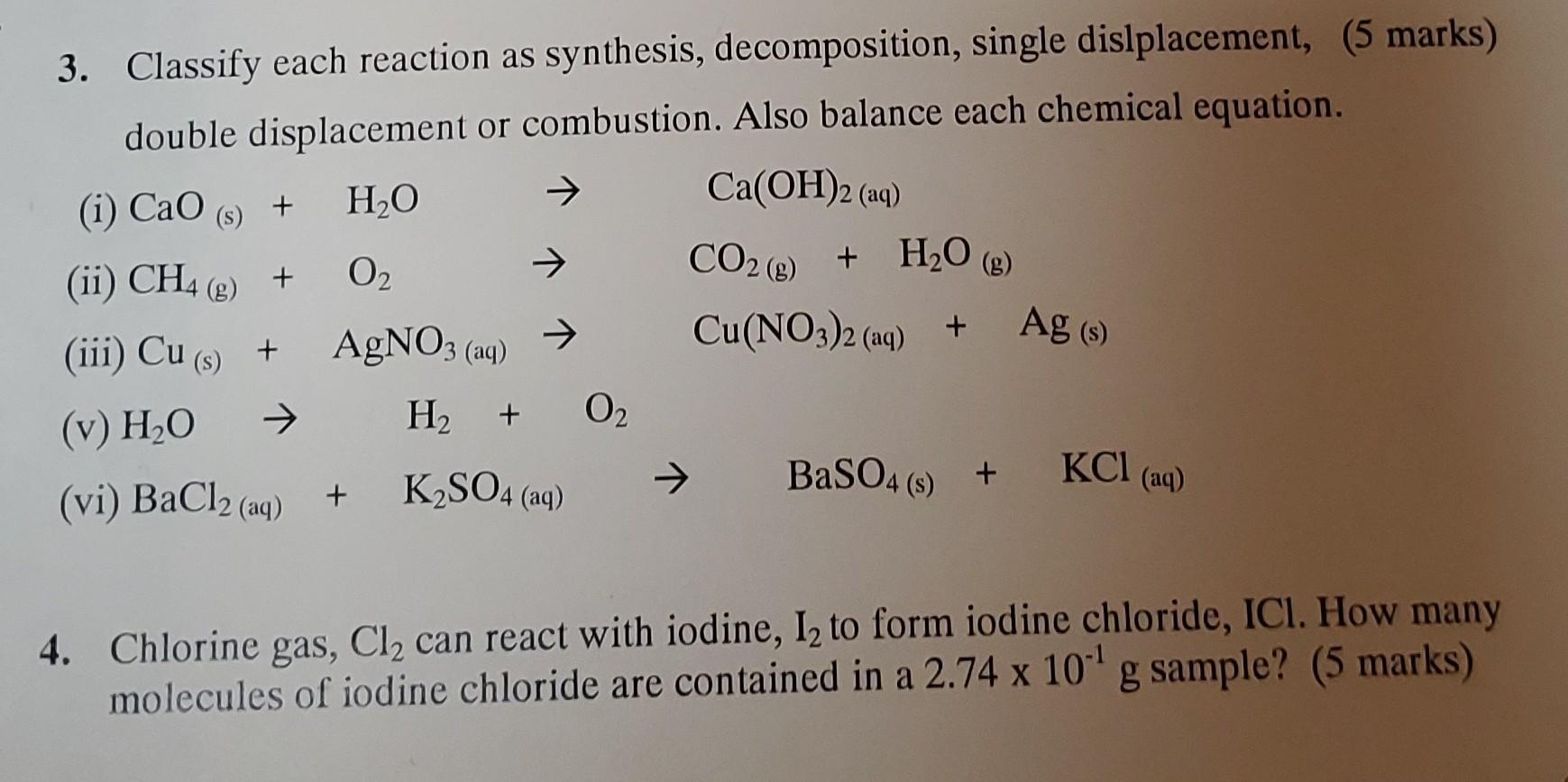
Question: 3,4 please 3. Classify each reaction as synthesis, decomposition, single dislplacement, (5 marks) double displacement or combustion. Also balance each chemical equation. (i) CaO(s)+H2OCa(OH)2(aq) (ii)
3,4 please
3. Classify each reaction as synthesis, decomposition, single dislplacement, (5 marks) double displacement or combustion. Also balance each chemical equation. (i) CaO(s)+H2OCa(OH)2(aq) (ii) CH4(g)+O2CO2(g)+H2O(g) (iii) Cu(s)+AgNO3(aq)Cu(NO3)2(aq)+Ag(s) (v) H2OH2+O2 (vi) BaCl2(aq)+K2SO4(aq)BaSO4(s)+KCl(aq) 4. Chlorine gas, Cl2 can react with iodine, I2 to form iodine chloride, ICl. How many molecules of iodine chloride are contained in a 2.74101g sample
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


