Question: 37 A electrolytic cell operating under standard conditions (1.0 M ion concentrations) utilizes the following reaction: 2Al3+(aq) + 3 Ni(s) = 2Al(s) + 3 Ni2+
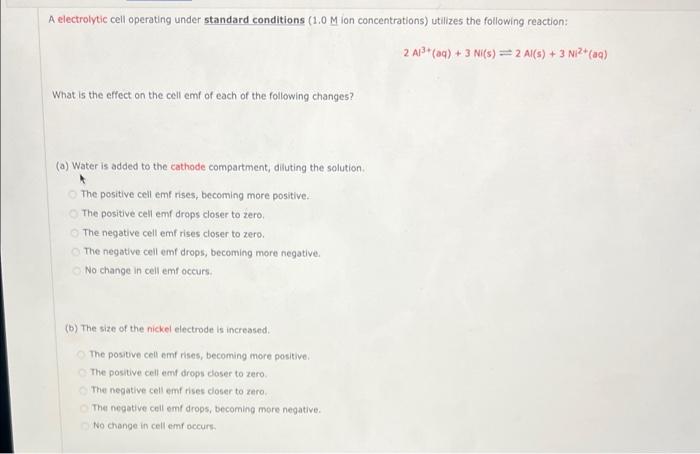

A electrolytic cell operating under standard conditions (1.0 M ion concentrations) utilizes the following reaction: 2Al3+(aq) + 3 Ni(s) = 2Al(s) + 3 Ni2+ (aq) What is the effect on the cell emf of each of the following changes? (a) Water is added to the cathode compartment, diluting the solution The positive cell emt rises, becoming more positive The positive cell emf drops closer to zero The negative cell emf rises closer to zero. The negative cell emf drops, becoming more negative No change in cell emf occurs (b) The size of the nickel electrode is increased The positive cell emf rises, becoming more positive The positive cell em drops doser to zero. The negative cellemfrises closer to zero The negative cell omf drops, becoming more negative No change in cellem occurs (C) A solution of 1.0 M NISO4 is added to the anode compartment. The positive cell emf rises, becoming more positive. The positive cell emf drops closer to zero. The negative cell emf rises closer to zero. The negative cell emf drops, becoming more negative. No change in cell emf occurs. (d) Some Na2S is added to the Al3+ compartment, precipitating some Al3+ as aluminum sulfide. The positive cell emf rises, becoming more positive. The positive cell emf drops closer to zero. The negative cell emf rises closer to zero. The negative cell emf drops, becoming more negative. No change in cell emf occurs
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


