Question: 3:nuary 31 OKINETICS AND EQUILIBRIUM Using reactant reaction order to predict changes in initial rate A certain reaction is second order in N, and first
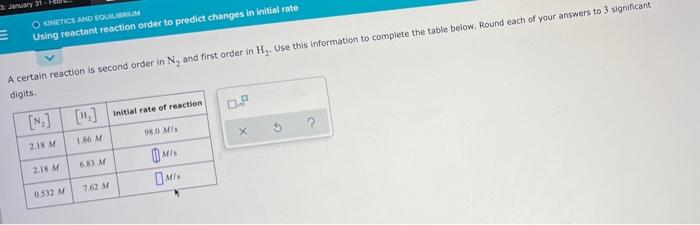
3:nuary 31 OKINETICS AND EQUILIBRIUM Using reactant reaction order to predict changes in initial rate A certain reaction is second order in N, and first order in Hz. Use this information to complete the table below. Round each of your answers to 3 significant digits 0. [N] [H] Initial rate of reaction ? 98.0 MB 1.86M 2.1 M 6.80 M 2.18 M DMS M 7.62 M 0.532 M
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


