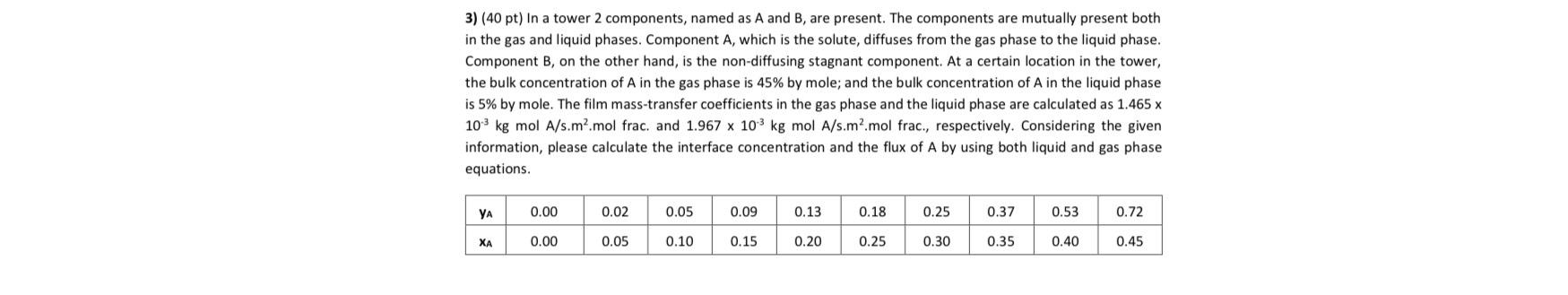
Question: ( 4 0 pt ) In a tower 2 components, named as A and B , are present. The components are mutually present both in
pt In a tower components, named as A and B are present. The components are mutually present both in the gas and liquid phases. Component A which is the solute, diffuses from the gas phase to the liquid phase. Component on the other hand, is the nondiffusing stagnant component. At a certain location in the tower, the bulk concentration of in the gas phase is by mole; and the bulk concentration of in the liquid phase is by mole. The film masstransfer coefficients in the gas phase and the liquid phase are calculated as kgmolmol frac. and kgmol mol frac., respectively. Considering the given information, please calculate the interface concentration and the flux of by using both liquid and gas phase equations.
table
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


