Question: ( 4 ) 1 . Niobium has an atomic radius of 0 . 1 4 3 0 n m and a density of 8 .
Niobium has an atomic radius of and a density of
Avogadros number atoms and atomic weight of
a Determine whether it has an FCC or BCC crystal structure?
b For which set of crystallographic planes will a firstorder diffraction peak occur at a
diffraction angle of when monochromatic ray radiation having a wavelength
of is used?
c Sketch one of the crystallographic planes of this set within a cubic unit cell that you
have found in part b
d Calculate the planar density of the plane you have drawn in part c in atoms
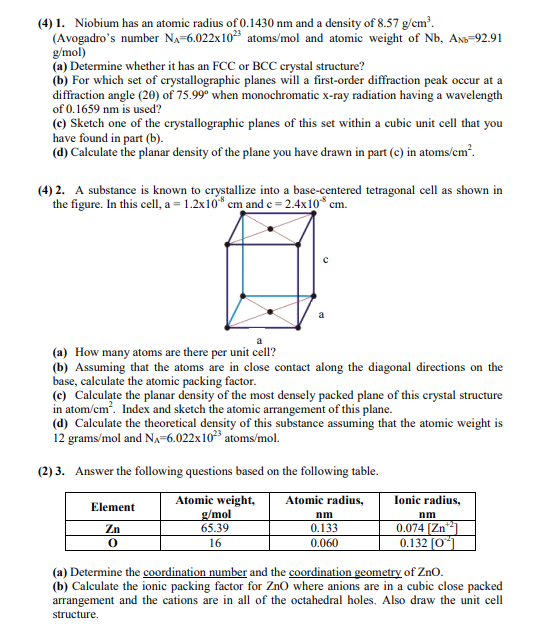
A substance is known to crystallize into a basecentered tetragonal cell as shown in
the figure. In this cell, and
a How many atoms are there per unit cell?
b Assuming that the atoms are in close contact along the diagonal directions on the
base, calculate the atomic packing factor.
c Calculate the planar density of the most densely packed plane of this crystal structure
in atom Index and sketch the atomic arrangement of this plane.
d Calculate the theoretical density of this substance assuming that the atomic weight is
gram and atoms
Answer the following questions based on the following table.
a Determine the coordination number and the coordination geometry of ZnO.
b Calculate the ionic packing factor for ZnO where anions are in a cubic close packed
arrangement and the cations are in all of the octahedral holes. Also draw the unit cell
structure.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


