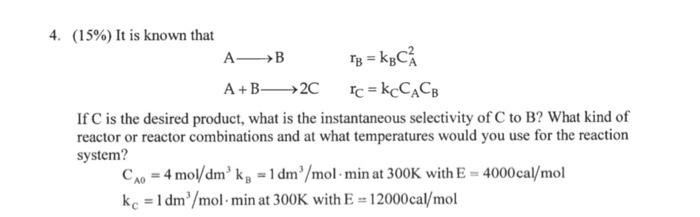
Question: 4. (15% It is known that A- _> B 13 = KBC? A + B- > 2C Ic = KCCACe If C is the desired
4. (15% It is known that
A- _> B
13 = KBC?
A + B- > 2C
Ic = KCCACe
If C is the desired product, what is the instantaneous selectivity of C to B? What kind of reactor or reactor combinations and at what temperatures would you use for the reaction system?
CAo = 4 mol/dm' kg = 1 dm' / mol- min at 300K with E = 4000cal/mol
kc =1dm mol min at 300K with E = 12000cal mol
4. (15%) It is known that ABA+B2CrB=kBCA2rC=kCCACB If C is the desired product, what is the instantaneous selectivity of C to B ? What kind of reactor or reactor combinations and at what temperatures would you use for the reaction system? CA0=4mol/dm3kB=1dm3/molminat300KwithE=4000cal/molkC=1dm3/molminat300KwithE=12000cal/mol 4. (15%) It is known that ABA+B2CrB=kBCA2rC=kCCACB If C is the desired product, what is the instantaneous selectivity of C to B ? What kind of reactor or reactor combinations and at what temperatures would you use for the reaction system? CA0=4mol/dm3kB=1dm3/molminat300KwithE=4000cal/molkC=1dm3/molminat300KwithE=12000cal/mol
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


