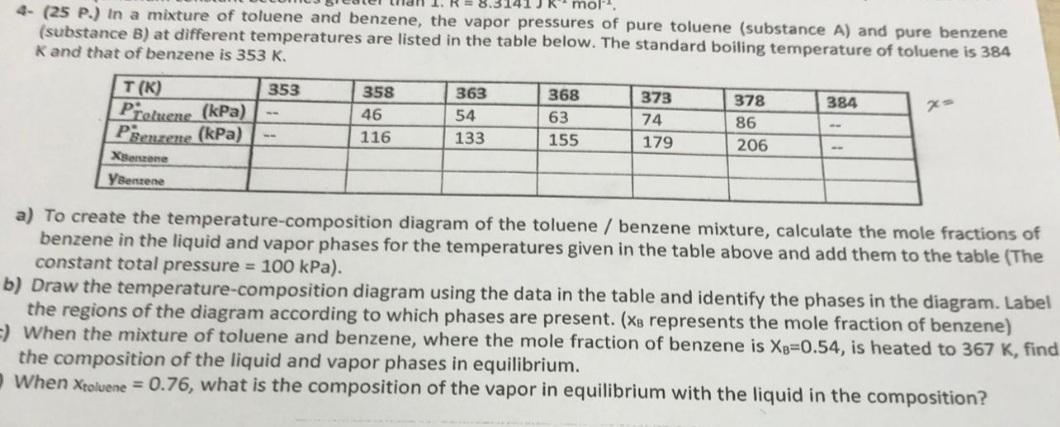
Question: 4 - ( 2 5 P . ) In a mixture of toluene and benzene, the vapor pressures of pure toluene ( substance A )
P In a mixture of toluene and benzene, the vapor pressures of pure toluene substance A and pure benzene substance B at different temperatures are listed in the table below. The standard bolling temperature of toluene is and that of benzene is
table
a To create the temperaturecomposition diagram of the toluene benzene mixture, calculate the mole fractions of benzene in the liquid and vapor phases for the temperatures given in the table above and add them to the table The constant total pressure kPa
b Draw the temperaturecomposition diagram using the data in the table and identify the phases in the diagram. Label the regions of the diagram according to which phases are present. represents the mole fraction of benzene
When the mixture of toluene and benzene, where the mole fraction of benzene is is heated to find the composition of the liquid and vapor phases in equilibrium.
When what is the composition of the vapor in equilibrium with the liquid in the composition?
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


