Question: #4. (20%) Analyze the NOx equilibrium by first constructing the corresponding van't Hoff graph and comparing it with the information available in Chapter 16 and

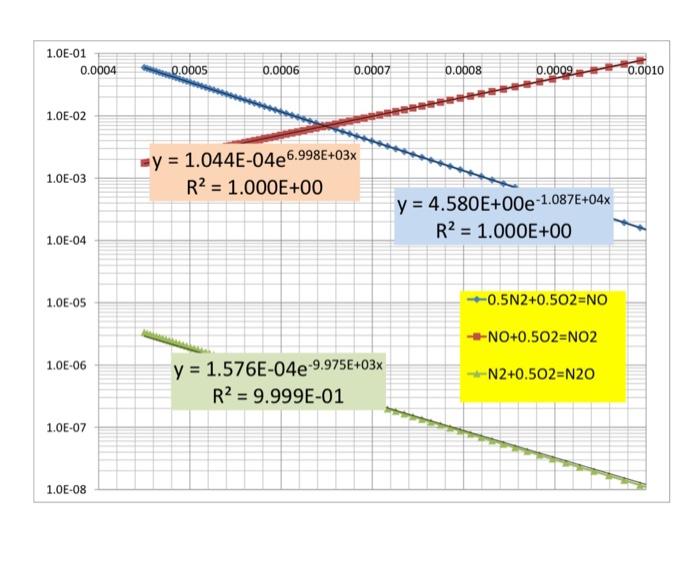
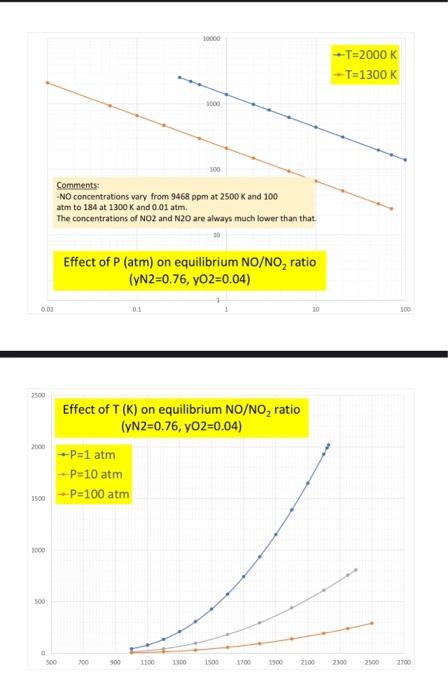
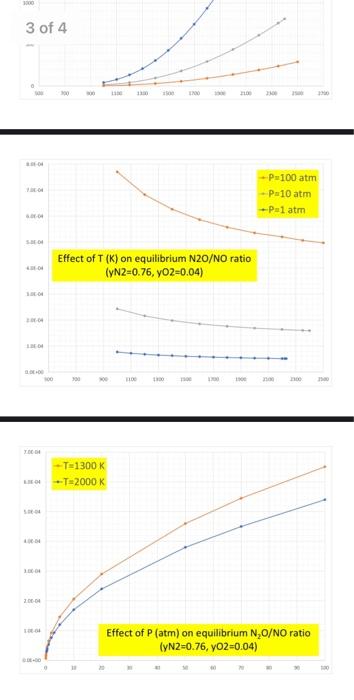
#4. (20%) Analyze the NOx equilibrium by first constructing the corresponding van't Hoff graph and comparing it with the information available in Chapter 16 and then summarizing the NO/NO2 (and N2O/NO?) dependence on P and T. Explain how this confirms, of course, the validity of the Le Chatelier principle. -Do some of your graphs look like this? Comments? (See also Q3 comments above...) 1.0E-01 0.0004 0.0005 0.0006 0.0007 0.0008 0.0009 0.0010 1.0E-02 1.0E-03 -y = 1.044E-04e6.998E+03x R2 = 1.000E+00 y = 4.580E+00e-1.087E+04x R2 = 1.000E+00 = 1.0E-04 1.0E-05 ---0.5N2+0.502=NO -NO+0.502-NO2 1.08-06 y = 1.576E-04e-9.975E+03x R2 = 9.999E-01 -N2+0.502-N20 = 1.0E-07 1.0E-08 10000 --T=2000 K -T=1300 K 3000 Comments: -NO concentrations vary from 9468 ppm at 2500 K and 100 atm to 184 at 1300 K and 0.01 atm. The concentrations of NO2 and N2O are always much lower than that Effect of P (atm) on equilibrium NO/NO, ratio (YN2=0.76, YO2=0.04) 0.01 10 300 2500 Effect of T (K) on equilibrium NO/NO, ratio (yN2=0.76, yO2=0.04) 2000 --P=1 atm P=10 atm 3800 --P=100 atm 3000 8 500 a 500 700 300 1100 1300 1500 1700 2100 2100 2500 2700 3 of 4 P100 atm -Pa 10 atm Palatm Effect of T (K) on equilibrium N20/NO ratio (YN2=0.76, yO2=0.04) 10 10 TO -T=1300 K 2000 K LE 200 Effect of P (atm) on equilibrium N,0/NO ratio (YN2=0.76, YO2=0.04) 30 #4. (20%) Analyze the NOx equilibrium by first constructing the corresponding van't Hoff graph and comparing it with the information available in Chapter 16 and then summarizing the NO/NO2 (and N2O/NO?) dependence on P and T. Explain how this confirms, of course, the validity of the Le Chatelier principle. -Do some of your graphs look like this? Comments? (See also Q3 comments above...) 1.0E-01 0.0004 0.0005 0.0006 0.0007 0.0008 0.0009 0.0010 1.0E-02 1.0E-03 -y = 1.044E-04e6.998E+03x R2 = 1.000E+00 y = 4.580E+00e-1.087E+04x R2 = 1.000E+00 = 1.0E-04 1.0E-05 ---0.5N2+0.502=NO -NO+0.502-NO2 1.08-06 y = 1.576E-04e-9.975E+03x R2 = 9.999E-01 -N2+0.502-N20 = 1.0E-07 1.0E-08 10000 --T=2000 K -T=1300 K 3000 Comments: -NO concentrations vary from 9468 ppm at 2500 K and 100 atm to 184 at 1300 K and 0.01 atm. The concentrations of NO2 and N2O are always much lower than that Effect of P (atm) on equilibrium NO/NO, ratio (YN2=0.76, YO2=0.04) 0.01 10 300 2500 Effect of T (K) on equilibrium NO/NO, ratio (yN2=0.76, yO2=0.04) 2000 --P=1 atm P=10 atm 3800 --P=100 atm 3000 8 500 a 500 700 300 1100 1300 1500 1700 2100 2100 2500 2700 3 of 4 P100 atm -Pa 10 atm Palatm Effect of T (K) on equilibrium N20/NO ratio (YN2=0.76, yO2=0.04) 10 10 TO -T=1300 K 2000 K LE 200 Effect of P (atm) on equilibrium N,0/NO ratio (YN2=0.76, YO2=0.04) 30
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


