Question: ...... ..... 4. A process to produce sulfuric acid roasts (burns) iron pyrites (FeS2) with air. The following reactions take place in the roasting furnace:
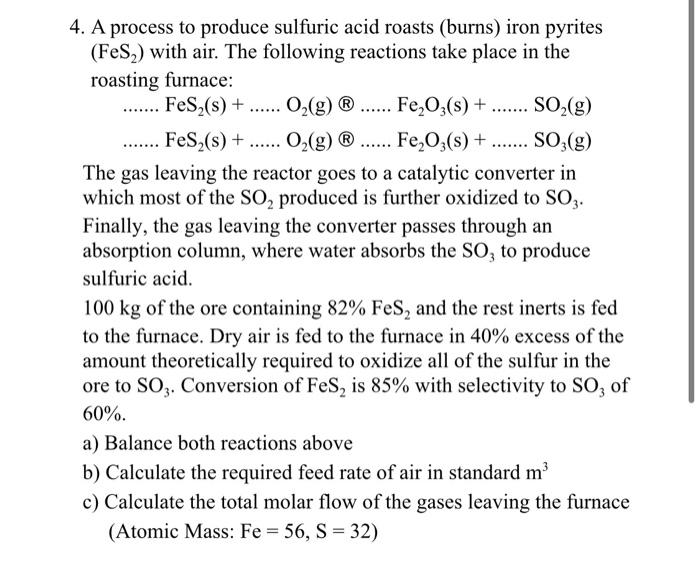
...... ..... 4. A process to produce sulfuric acid roasts (burns) iron pyrites (FeS2) with air. The following reactions take place in the roasting furnace: .... FeS2(s) + O2(g) Fe,O3(s) + ....... SO2(g) FeS (s) + O2(g) Fe,O3(s) + ....... SO3(g) The gas leaving the reactor goes to a catalytic converter in which most of the SO2 produced is further oxidized to SOZ. Finally, the gas leaving the converter passes through an absorption column, where water absorbs the SO, to produce sulfuric acid. 100 kg of the ore containing 82% Fes, and the rest inerts is fed to the furnace. Dry air is fed to the furnace in 40% excess of the amount theoretically required to oxidize all of the sulfur in the ore to SO3. Conversion of Fes, is 85% with selectivity to SO, of 60%. a) Balance both reactions above b) Calculate the required feed rate of air in standard m c) Calculate the total molar flow of the gases leaving the furnace (Atomic Mass: Fe = 56, S = 32)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


