The quantity of sulfuric acid used globally places it among the most plentiful of all commodity chemicals.
Question:
The quantity of sulfuric acid used globally places it among the most plentiful of all commodity chemicals. In the modern chemical industry, synthesis of most sulfuric acid utilizes elemental sulfur as a feedstock. However, an alternative and historically important source of sulfuric acid was the conversion of an ore containing iron pyrites (FeS2) to sulfur oxides by roasting (burning) the ore with air. The following reactions occurred in an oven:
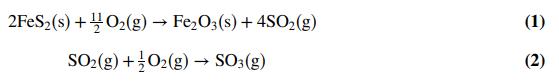
The gases leaving the oven were fed to a catalytic converter in which most of the remaining SO2 produced was oxidized to SO3. Finally, the gas leaving the converter was sent to an absorption column where the SO3 was taken up by water to produce sulfuric acid (H2SO4). (a) The ore fed to the oven was 90.0 wt% FeS2, and the remaining material may be considered inert. Dry air was fed to the oven in 30.0% excess of the amount required to oxidize all of the sulfur in the ore to SO3. Eighty-five percent of the FeS2 was oxidized, and 60% of the SO2 produced was oxidized to SO3. Leaving the roaster were (i) a gas stream containing SO2 , SO3 , O2 , and N2 and (ii) a solid stream containing unconverted pyrites, ferric oxide (Fe2O3), and the inert material. Calculate the required feed rate of air in standard cubic meters per 100 kg of ore fed to the process. Also determine the molar composition and volume (SCM/100 kg ore) of the gas leaving the oven.
(b) The gas leaving the oven entered the catalytic converter, which operated at 1.0 atm. Reaction (2) proceeded to equilibrium, at which point the component partial pressures are related by the expression
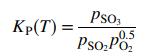
The gases were first heated to 600°C to accelerate the rate of reaction, and then cooled to 400°C to enhance SO2 conversion. The equilibrium constant KP at these two temperatures is 9:53 atm0.5 and 397 atm0.5 , respectively. Calculate the equilibrium fractional conversions of SO2 at these two temperatures.
(c) Estimate the production rate of sulfuric acid in kg/kg ore if all of the SO3 leaving the converter was transformed to sulfuric acid. What would this value be if all the sulfur in the ore had been converted?
Exploratory Exercises—Research and Discover
(d) Two of the important factors affecting the utility of a chemical reaction are the maximum extent of the reaction and the rate at which the reaction occurs. Consider these two factors and explain the steps in the converter in which the gas was heated first and then cooled.
(e) Why has elemental sulfur come to be the dominant feedstock in sulfuric acid manufacturing?
Step by Step Answer:

Elementary Principles of Chemical Processes
ISBN: 978-1119498759
4th edition
Authors: Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard





