Question: 4. An adiabatic separation process is shown in the figure below. Determine the enthalpy of stream 4 (H4) in kJ/kmol. State necessary assumptions. (10 points)
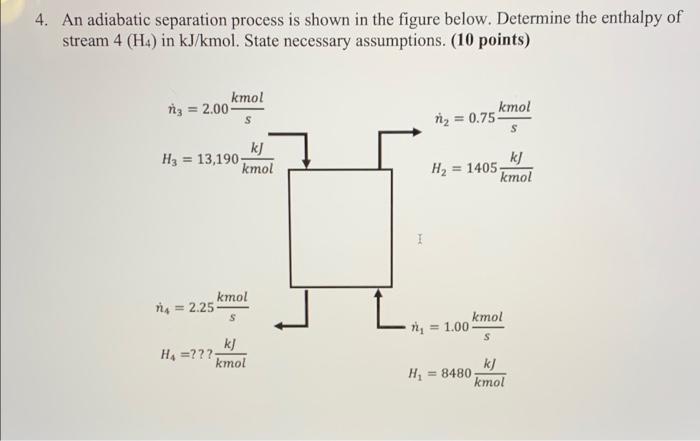
4. An adiabatic separation process is shown in the figure below. Determine the enthalpy of stream 4 (H4) in kJ/kmol. State necessary assumptions. (10 points) kmol n = 2.00 kmol niz = 0.75 kJ H3 = 13,190 kmol kej H2 = 1405 kmol 1 kmol n = 2.25 S kmol 100= k) HA=??? kmol k] Hi = 8480 kmol
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


