Question: 4. Consider the first phase transition in Balioz. Below the phase transition cer- tain ions are displaced parallel to each other forming an electrically polar
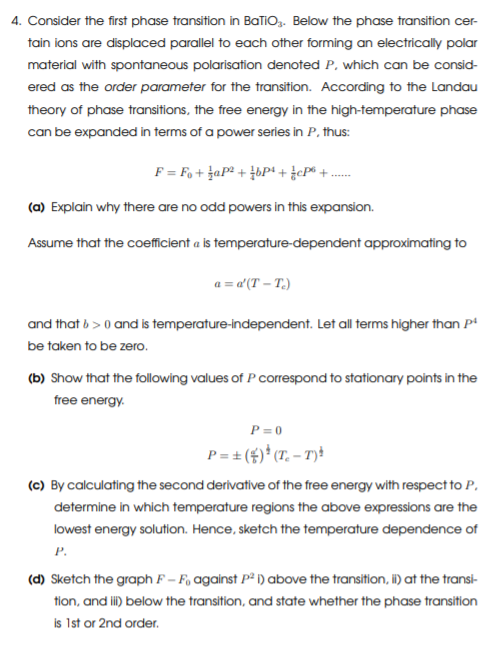
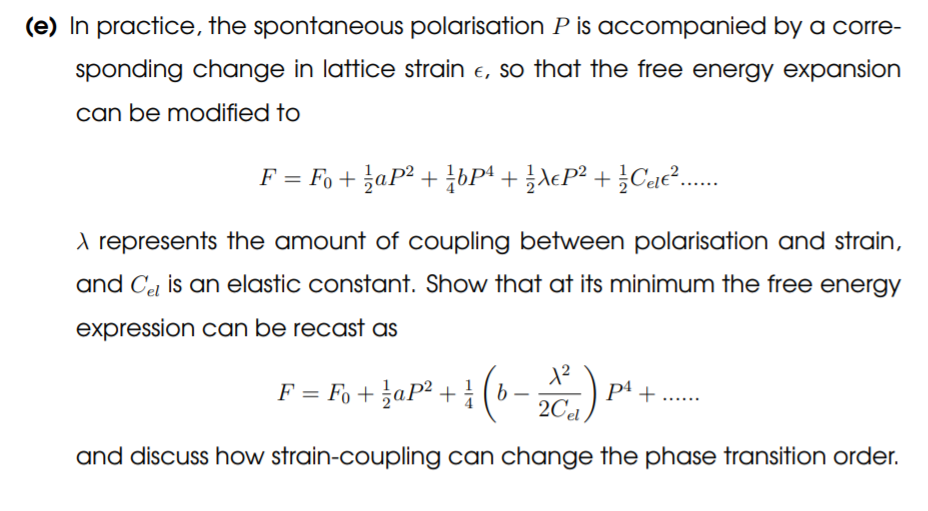
4. Consider the first phase transition in Balioz. Below the phase transition cer- tain ions are displaced parallel to each other forming an electrically polar material with spontaneous polarisation denoted P, which can be consid- ered as the order parameter for the transition. According to the Landau theory of phase transitions, the free energy in the high-temperature phase can be expanded in terms of a power series in P, thus: F = Fo+ aP2 + 33P+ cP6 + (a) Explain why there are no odd powers in this expansion Assume that the coefficient a ls temperature-dependent approximating to a = d'(T-T.) and that b>0 and is temperature-independent. Let all terms higher than p be taken to be zero. (b) Show that the following values of P correspond to stationary points in the free energy P=0 P=+($)*(T.-T): (c) By calculating the second derivative of the free energy with respect to P. determine in which temperature regions the above expressions are the lowest energy solution. Hence, sketch the temperature dependence of P. (d) Sketch the graph F - F, against P ) above the transition, i) at the transi- tion, and ii) below the transition, and state whether the phase transition is 1st or 2nd order. (e) In practice, the spontaneous polarisation P is accompanied by a corre- sponding change in lattice strain e, so that the free energy expansion can be modified to F = Fo + aP2 + {bP4 +}{cP2 +}Cele?...... 1 represents the amount of coupling between polarisation and strain, and Ce is an elastic constant. Show that at its minimum the free energy expression can be recast as 12 F = Fo + zap2 + ( 6 2Cel pt + and discuss how strain-coupling can change the phase transition order
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


