Question: 4. Consider the proton shown on each molecule below, and select the statement that best explains which compound is more acidic. A. Compound II is
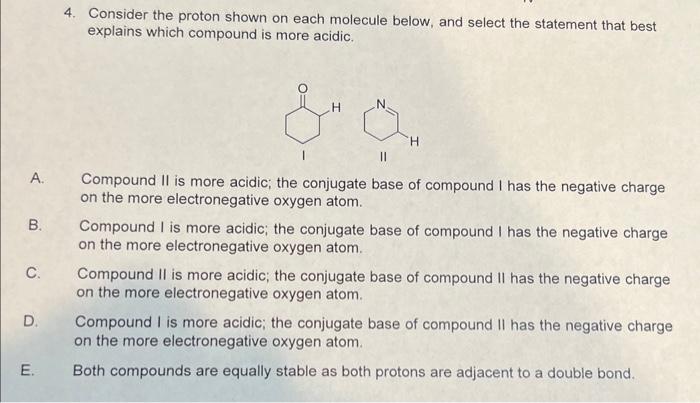
4. Consider the proton shown on each molecule below, and select the statement that best explains which compound is more acidic. A. Compound II is more acidic; the conjugate base of compound I has the negative charge on the more electronegative oxygen atom. B. Compound I is more acidic; the conjugate base of compound I has the negative charge on the more electronegative oxygen atom. C. Compound II is more acidic; the conjugate base of compound II has the negative charge on the more electronegative oxygen atom. D. Compound I is more acidic; the conjugate base of compound II has the negative charge on the more electronegative oxygen atom. E. Both compounds are equally stable as both protons are adjacent to a double bond
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


