Question: 4. Consider these two reactions: Reaction 1: NO2 + hu NO + 0 ki Reaction 2: H02 + NO NO2 + OH k2 The reaction
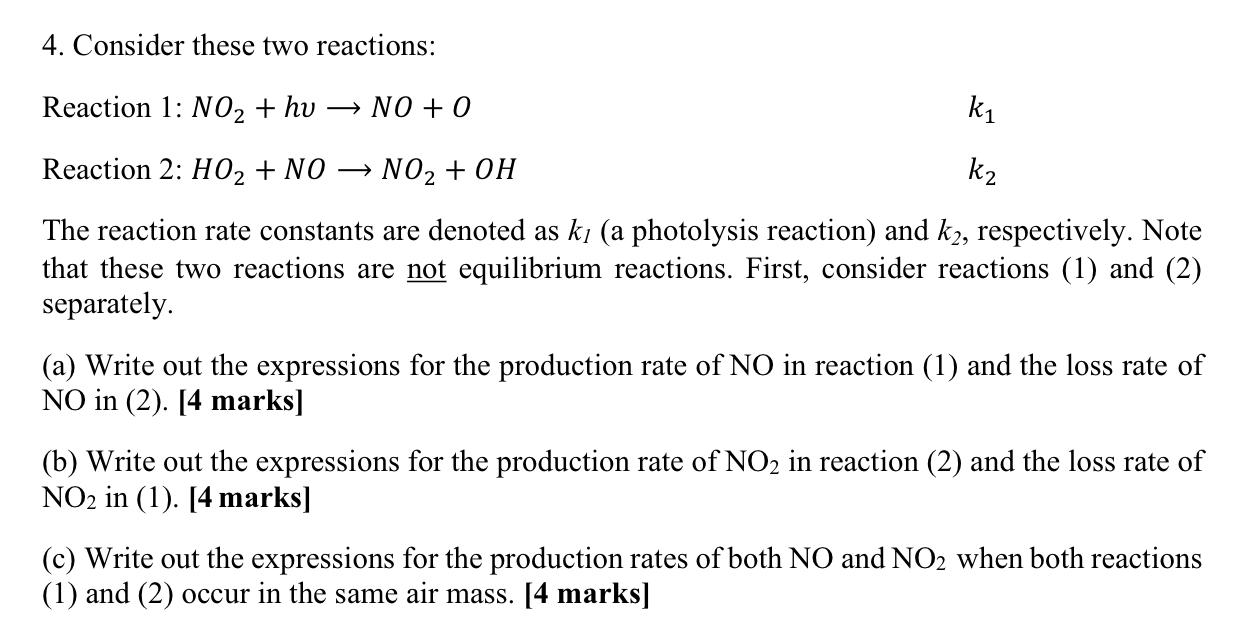
4. Consider these two reactions: Reaction 1: NO2 + hu NO + 0 ki Reaction 2: H02 + NO NO2 + OH k2 The reaction rate constants are denoted as ki (a photolysis reaction) and k2, respectively. Note that these two reactions are not equilibrium reactions. First, consider reactions (1) and (2) separately. (a) Write out the expressions for the production rate of NO in reaction (1) and the loss rate of NO in (2). [4 marks] (b) Write out the expressions for the production rate of NO2 in reaction (2) and the loss rate of NO2 in (1). [4 marks] (c) Write out the expressions for the production rates of both NO and NO2 when both reactions (1) and (2) occur in the same air mass. [4 marks]
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


