Question: 4. Determining appropriate rate expression given two possible rate mechanisms. While drinking water disinfection has decreased waterborne diseases, it has also let to the formation
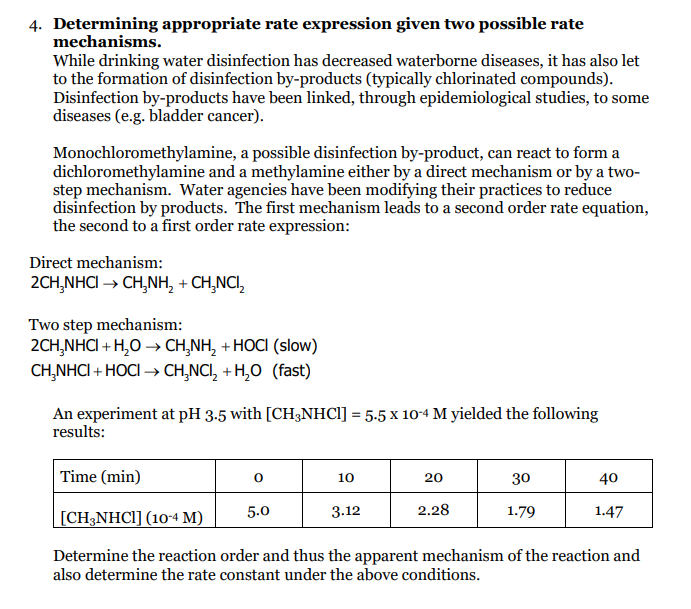
4. Determining appropriate rate expression given two possible rate mechanisms. While drinking water disinfection has decreased waterborne diseases, it has also let to the formation of disinfection by-products (typically chlorinated compounds). Disinfection by-products have been linked, through epidemiological studies, to some diseases (e.g. bladder cancer). Monochloromethylamine, a possible disinfection by-product, can react to form a dichloromethylamine and a methylamine either by a direct mechanism or by a two- step mechanism. Water agencies have been modifying their practices to reduce disinfection by products. The first mechanism leads to a second order rate equation, the second to a first order rate expression: Direct mechanism: 2CH NHC CHNH, + CHCNC, Two step mechanism: 2CH NHCI +H2O CH_NH, +HOCI (slow) CH NHCI + HOCI CH NCI, +H,0 (fast) An experiment at pH 3.5 with [CH3NHCl] = 5.5 x 10-4 M yielded the following results: Time (min) 0 10 20 30 40 5.0 [CH3NHCl] (10-4 M) 3.12 2.28 1.79 1.47 Determine the reaction order and thus the apparent mechanism of the reaction and also determine the rate constant under the above conditions
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


