Question: a) Given the training constants and E for Fe/Fe* equal to 0.771V Fe+ Y+ ++ FeY Fe++ Y+ + FeY2- KF = 1,3 1025
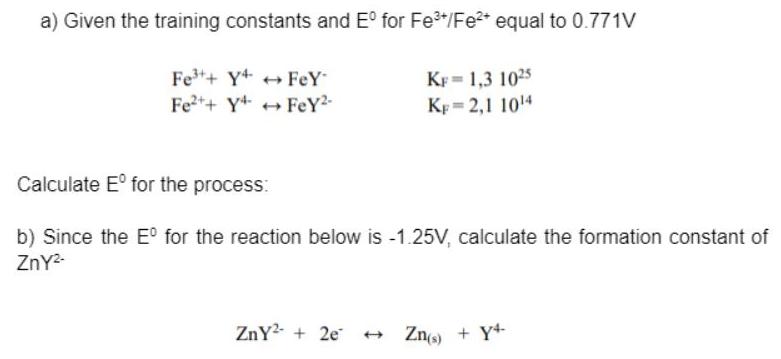
a) Given the training constants and E for Fe"/Fe* equal to 0.771V Fe+ Y+ ++ FeY Fe++ Y+ + FeY2- KF = 1,3 1025 Kp = 2,1 1014 Calculate E for the process: b) Since the E for the reaction below is -1.25V, calculate the formation constant of ZnY- ZnY2- + 2e + Zno) + Y+
Step by Step Solution
3.41 Rating (154 Votes )
There are 3 Steps involved in it
sclutio o Given as Fe Fe 0771V Fe3t te f0771V Comvesting Eo the Standard potenti al for ... View full answer

Get step-by-step solutions from verified subject matter experts


