Question: 4. For a linear condensation reaction of A- A- and B-B-type monomers A. Calculate the extent of reaction (p) nec- essary to achieve a number
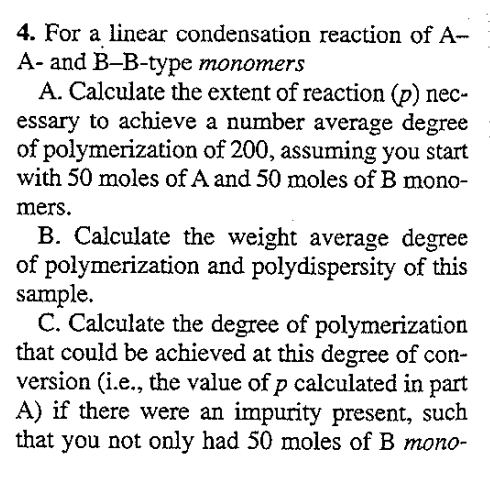
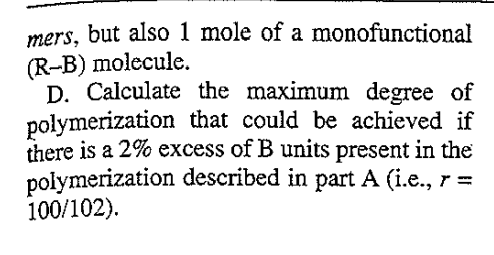
4. For a linear condensation reaction of A- A- and B-B-type monomers A. Calculate the extent of reaction (p) nec- essary to achieve a number average degree of polymerization of 200, assuming you start with 50 moles of A and 50 moles of B mono- mers. B. Calculate the weight average degree of polymerization and polydispersity of this sample. C. Calculate the degree of polymerization that could be achieved at this degree of con- version (i.e., the value of p calculated in part A) if there were an impurity present, such that you not only had 50 moles of B mono- mers, but also 1 mole of a monofunctional (R-B) molecule. D. Calculate the maximum degree of polymerization that could be achieved if there is a 2% excess of B units present in the polymerization described in part A (i.e., r = 100/102)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


