Question: 4. Generate the reducible representation for the four p orbitals of B atoms (it is not boron) in the square planar AB4 molecule using the
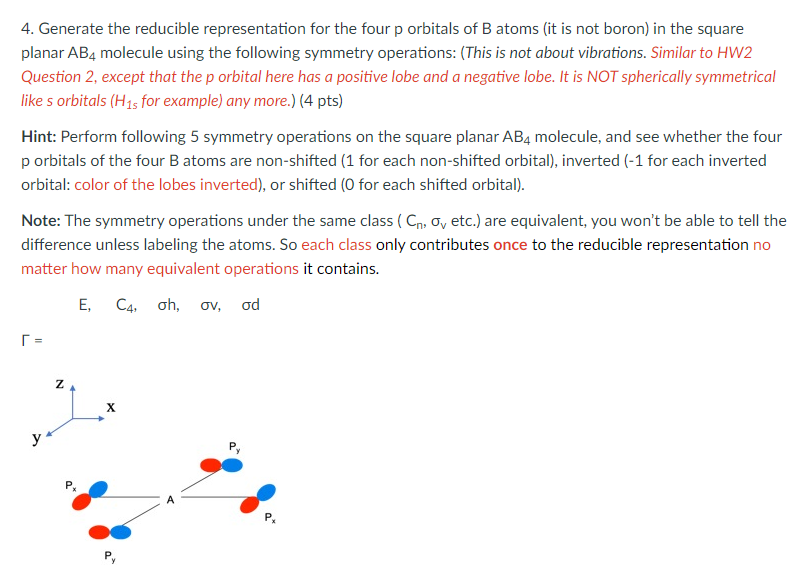
4. Generate the reducible representation for the four p orbitals of B atoms (it is not boron) in the square planar AB4 molecule using the following symmetry operations: (This is not about vibrations. Similar to HW2 Question 2, except that the p orbital here has a positive lobe and a negative lobe. It is NOT spherically symmetrical like s orbitals (H15 for example) any more.) (4 pts) Hint: Perform following 5 symmetry operations on the square planar AB4 molecule, and see whether the four p orbitals of the four B atoms are non-shifted (1 for each non-shifted orbital), inverted (-1 for each inverted orbital: color of the lobes inverted), or shifted (O for each shifted orbital). Note: The symmetry operations under the same class (Co, Ov etc.) are equivalent, you won't be able to tell the difference unless labeling the atoms. So each class only contributes once to the reducible representation no matter how many equivalent operations it contains. E, C4. oh, ov, od N z X y P a
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


