Question: 4) Given the equation and Kc below. CH:CH OH(g) + CH4(g) + H2O(g) Kc = 1.10X10-3 a) What concentration of ethanol CH3CH2OH must be heated
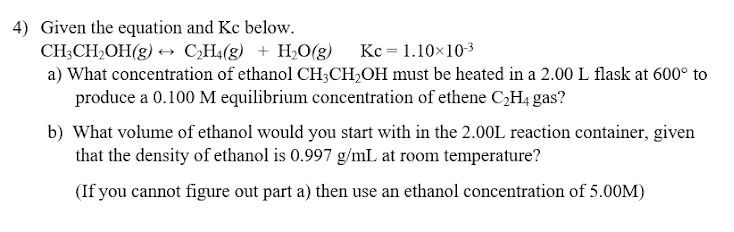
4) Given the equation and Kc below. CH:CH OH(g) + CH4(g) + H2O(g) Kc = 1.10X10-3 a) What concentration of ethanol CH3CH2OH must be heated in a 2.00 L flask at 600 to produce a 0.100 M equilibrium concentration of ethene CH4 gas? b) What volume of ethanol would you start with in the 2.00L reaction container, given that the density of ethanol is 0.997 g/mL at room temperature? (If you cannot figure out part a) then use an ethanol concentration of 5.00M)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


