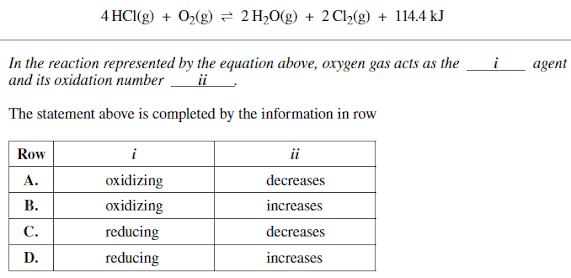
Question: 4 H C l ( g ) + O 2 ( g ) 2 H 2 O ( g ) + 2 C l 2
In the reaction represented by the equation above, oxygen gas acts as the
agent
and its oxidation number
ii
The statement above is completed by the information in row
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


