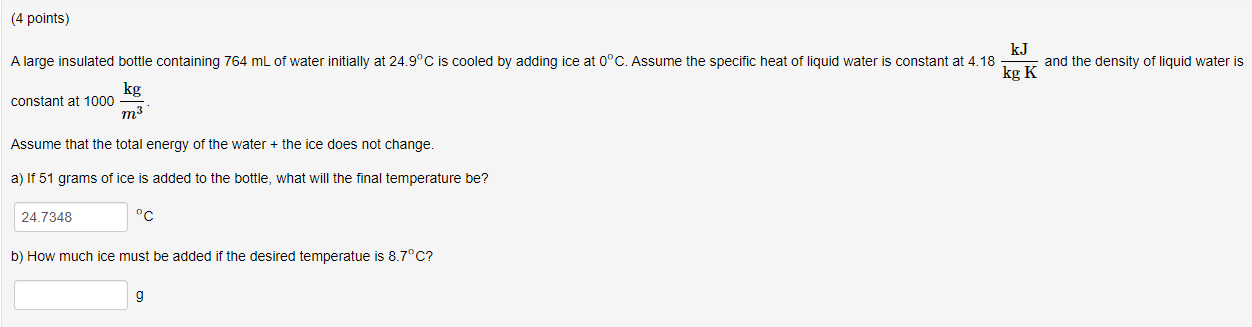
Question: ( 4 points ) A large insulated bottle containing 7 6 4 mL of water initially at 2 4 . 9 C is cooled by
points
A large insulated bottle containing mL of water initially at is cooled by adding ice at Assume the specific heat of liquid water is constant at and the density of liquid water is
constant at
Assume that the total energy of the water the ice does not change.
a If grams of ice is added to the bottle, what will the final temperature be
b How much ice must be added if the desired temperatue is
g
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


