Question: (4 points) Styrene monomer is polymerized ionically in dichloroethane at a concentration of 200 g/dm. H2SO4 is used as the initiator at a 20C reaction
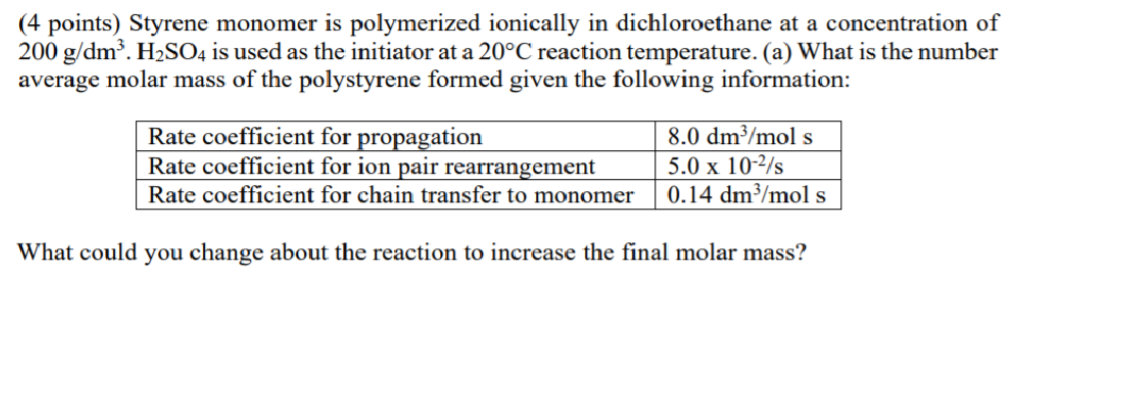
(4 points) Styrene monomer is polymerized ionically in dichloroethane at a concentration of 200 g/dm. H2SO4 is used as the initiator at a 20C reaction temperature. (a) What is the number average molar mass of the polystyrene formed given the following information: Rate coefficient for propagation 8.0 dm3/mol s Rate coefficient for ion pair rearrangement 5.0 x 10-2/s Rate coefficient for chain transfer to monomer 0.14 dm3/mol s What could you change about the reaction to increase the final molar mass
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


