Question: 4. The hypothetical molecule ML5 shown below has a trigonal bipyramidal structure. All M-L bond lengths are equal. Answer the following questions regarding the symmetry
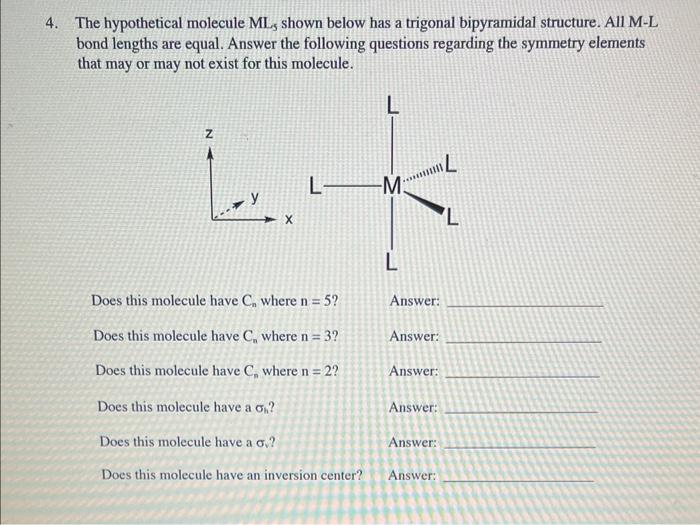
4. The hypothetical molecule ML5 shown below has a trigonal bipyramidal structure. All M-L bond lengths are equal. Answer the following questions regarding the symmetry elements that may or may not exist for this molecule. Does this molecule have Cn where n=5 ? Answer: Does this molecule have Cn where n=3 ? Answer: Does this molecule have Cn where n=2 ? Answer: Does this molecule have a h ? Answer: Does this molecule have a v ? Answer: Does this molecule have an inversion center
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


