Question: 4. The relationship between the pressure drop per unit length, AP2 (kPa m.) along a smooth-walled, horizontal pipe and the variables that affect the pressure
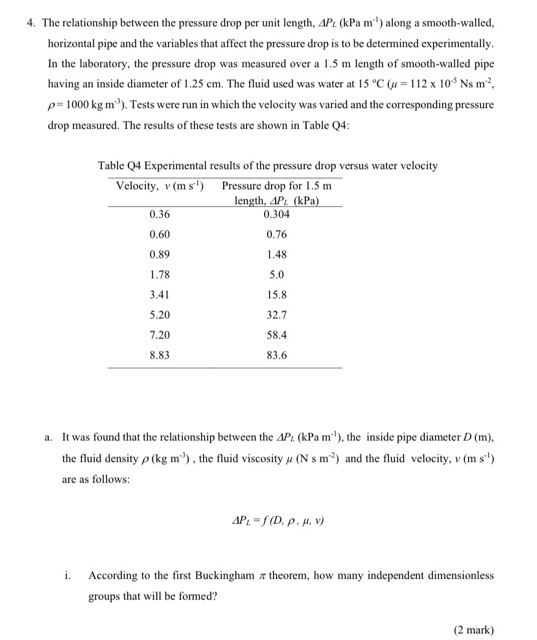
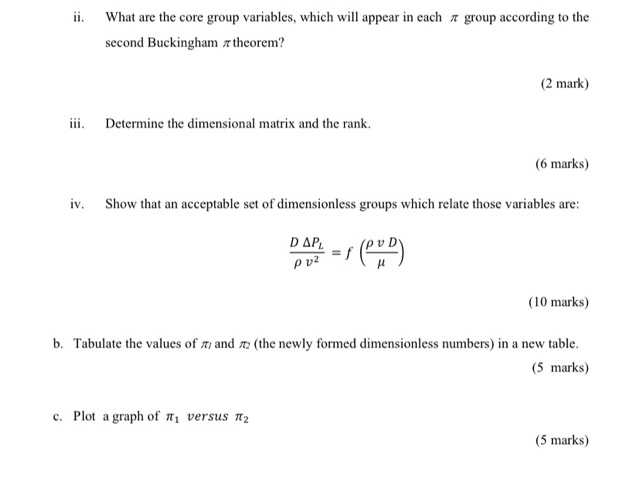
4. The relationship between the pressure drop per unit length, AP2 (kPa m.) along a smooth-walled, horizontal pipe and the variables that affect the pressure drop is to be determined experimentally. In the laboratory, the pressure drop was measured over a 1.5 m length of smooth-walled pipe having an inside diameter of 1.25 cm. The fluid used was water at 15 C (u = 112 x 109Ns m? p=1000 kg m). Tests were run in which the velocity was varied and the corresponding pressure drop measured. The results of these tests are shown in Table 04: Table Q4 Experimental results of the pressure drop versus water velocity Velocity, v (ms) Pressure drop for 1.5 m length, AP (kPa) 0.36 0.304 0.60 0.76 0.89 1.48 1.78 3.41 15.8 5.20 32.7 7.20 58.4 5.0 8.83 83.6 a. It was found that the relationship between the AP (kPa m"), the inside pipe diameter D (m), the fluid density p (kg m), the fluid viscosity (N sm) and the fluid velocity, v (m s') are as follows: AP= S(D. p. 4.) i. According to the first Buckingham # theorem, how many independent dimensionless groups that will be formed? (2 mark) ii. What are the core group variables, which will appear in each group according to the second Buckingham #theorem? (2 mark) iii. Determine the dimensional matrix and the rank. (6 marks) iv. Show that an acceptable set of dimensionless groups which relate those variables are: D . = f ( CD) ? (10 marks) b. Tabulate the values of nei and 72 (the newly formed dimensionless numbers) in a new table. (5 marks) c. Plot a graph of t, versus Tt2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


