Question: 4. Theoretical Yield Calculation: (5 points). Using the balanced chemical reaction and amounts of reagents for the Fischer Esterification reaction found in your lab guide,

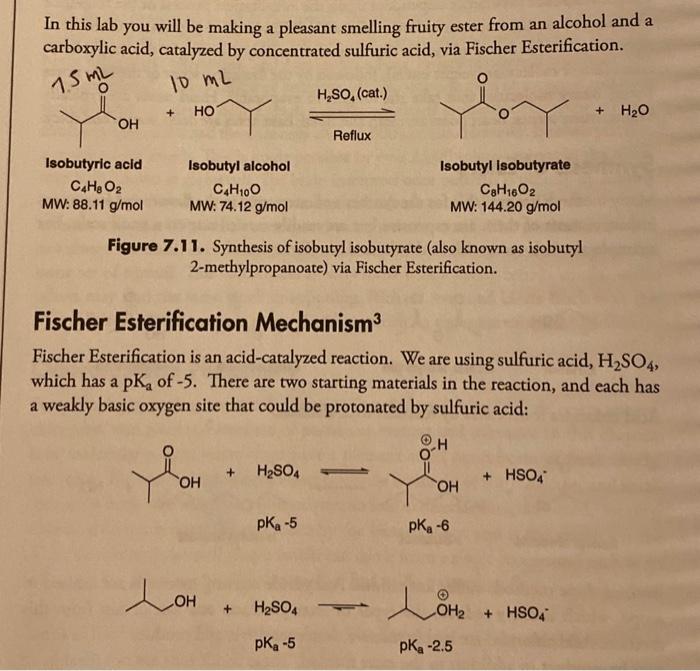
4. Theoretical Yield Calculation: (5 points). Using the balanced chemical reaction and amounts of reagents for the Fischer Esterification reaction found in your lab guide, calculate the theoretical yield for the ester in grams and mL using the limiting reagent. [NOTE: show cales for both the carboxylic acid and alcohol starting materials]. Place the mmol/mL/gram quantity for the product in your chemical data table in Question $2. In this lab you will be making a pleasant smelling fruity ester from an alcohol and a carboxylic acid, catalyzed by concentrated sulfuric acid, via Fischer Esterification. Isobutyric acid Isobutyl alcohol Isobutyl isobutyrate C4H8O2 MW: 88.11g/mol C4H10O MW: 74.12g/mol C8H16O2 MW: 144.20g/mol Figure 7.11. Synthesis of isobutyl isobutyrate (also known as isobutyl 2-methylpropanoate) via Fischer Esterification. Fischer Esterification Mechanism 3 Fischer Esterification is an acid-catalyzed reaction. We are using sulfuric acid, H2SO4, which has a pK2 of 5. There are two starting materials in the reaction, and each has a weakly basic oxygen site that could be protonated by sulfuric acid
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


